
Fig.1 Open cultivation technique: (a) Paddle wheel raceway pond. (b) Circular pond (SundarRajan et al., 2019).
David Osei-Wusu1, Pengfei Cheng2*, Yan Wang2, Chengxu Zhou2, Tianzhong Liu3, Shuhao Huo1*
1. School of Food and Biological Engineering, Jiangsu University, Zhenjiang 212013, China
2. College of Food and Pharmaceutical Sciences, Ningbo University, Ningbo 315211, China
3. Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, Qingdao266101, China
* Corresponding author.
Pengfei Cheng, Tel: +86 13819423131; E-mail: pfcheng1792@163.com
Shuhao Huo, Tel: +86 18851280536; E-mail:huoshuhao@yeah.net
Received 13-11-2018; received in revised form 11-02-2019; accepted 18-02-2019
Abstract Swine wastewater significantly contains high levels of nutrients and toxic pollutants that have an impact on the ecosystem and public health. This review identifies and discusses the opportunities and challenges of different methods of treating swine wastewater. Although various conventional ways have been used in treating swine wastewater as described in literature, they are mostly employed to aid in further biological advanced swine wastewater treatment processes. The used of microalgae appear to be a successful treatment for nutrient-rich swine wastewater. Presently, microalgae wastewater treatment and bioproduct production depend mostly on suspended algal culture and it has been limited by low productivity of algal biomass, high operation cost, and high-energy consumption in harvesting algal cells. For this reason, in this review, we discussed how biofilm attached cultivation methods are developed to overcome recent limitations in swine wastewater treatment. Microalgae biomass productivity using the biofilm-attached method could reach 2.8 times higher than that of the suspended system for bioproducts productions that are commonly described in literature. This review provides useful information for developing swine wastewater treatment with microalgal biomass production facility and for establishing an efficient cultivation of microalgae in a biofilm attached system.
Keywords: swine wastewater; treatment; microalga; biofilm-attached cultivation
Introduction
Intensive livestock industries continuously produce large amount of waste worldwide; signify progressively concerning danger to the environment, particularly if disposed of the waste inappropriately. Large amounts of water used for intensive livestock farming purposes arise to the production of large volumes of wastewater generated. Swine wastewater contains high amounts of chemical oxygen demand (COD), total nitrogen (TN), ammonium (NH4+), total phosphorus (TP), and suspended solids, which deteriorates freshwater ecology and thus is considered a public health threat to the immediate inhabitants in the locality of the discharging area (Ayre et al. 2017; Cai et al. 2013). High concentrations of NH4+ in water bodies can be converted to NO3-, which can be poisonous to aquatic organisms and finally to human beings. This obviously demands for effective management techniques capable of reducing nitrogen and phosphorus concentrations in wastewaters before being released. The standard in wastewater treatment is the decrease of nutrient and toxic metal to satisfactory levels prior to release and reuse (Miranda et al. 2017).
A conventional wastewater treatment removes nitrogen and phosphorus from wastewater in two separate methods. In the first, nitrogen is usually converted into N2 gas through coupled nitrification-identification, whereas phosphorus is precipitated with metal salts. In the second, nitrogen and phosphorus removal is attained in a chemical manner, such as precipitation using aluminum and iron salts. However, these methods are expensive and create a large amount of sludge contaminated with chemical compounds, demanding for additional treatment. Typically, biological processes are recognized as the utmost cost-effective method to treat swine wastewater (Cooper et al. 1994).Moreover, biological processes are totally influenced by the high concentration of ammoniac nitrogen present in swine wastewater, which can inhibit the activity of microorganisms (Vadivelu et al. 2006).
In the last decades, numerous studies have stated the prospective of microalgal consortia in diverse applications, including production of biomass and nutrient removal from wastewaters (Ramanan et al. 2016, Rawat et al. 2011). Microalgae need large quantities of nitrogen and phosphorus for their growth (Renuka et al. 2013), implying that these microorganisms can successfully uptake nitrogen and phosphorus from wastewaters. Actually, high nitrogen and phosphorus removal efficiencies (80%-100%) from wastewaters of different sources (e.g., agricultural, industrial and municipal ones) have previously been stated for microalgae (Zhu et al. 2013, Li et al. 2011). Kothari et al. (2012) found that Chlorella pyrenoidosa could remove about 80%-85% total phosphorus (TP) and 60%-80% of total nitrogen (TN) from dairy wastewater. Markou et al. (2012) also found that the maximum removal of chemical oxygen demand (COD) was 73.18%, while phenols, phosphorus, and nitrates in some cases were completely removed by cultivating Arthrospira platensis in olive-oil mill wastewater. Botryococcus braunii could successfully remove 93.3% and 40.8% of the TP and TN, respectively, with algal biofuel content of 23.8% in aerated swine lagoon wastewater cultivation (Liu et al. 2013a). Numerous studies have established that microalgae can be used as feedstock for bioenergy production, comprising of biodiesel, biomethane and biohydrogen (Kadir et al., 2018). Microalgae collected from swine wastewater show promising assets for biofuel production due to its tolerance and high growth rate in swine wastewater to produce good biomass, as well as high lipid and carbohydrate productivities (Cheng et al., 2019).
This paper reviews and over views the use of conventional methods of swine wastewater treatment and the respective limitations, and answers the reason why microalgae are used to remediate wastewaters for bioproducts productions. It also focuses on microalgae ability to remediate and biomass production in swine wastewater with treatment methods such as the use of open pond, closed system, and biofilm-attached cultivation systems.
Conventional ways of swine wastewater treatment
To implement tariffs and regulations on sanitation and wastewater treatment, as well as for disposal or reuse of treated effluents, it is necessary to know the conventional treatment technologies. Wastewater treatment has been evolving at a different pace along the history, according to the different contaminated water. The conventional methods of treating swine wastewater are shown in the Table 1.
Solid-liquid separation
This is the process of separating solid fraction from liquid fraction in swine wastewater. This method is often referred to as a pre-treatment stage of swine slurry before further treatment. Separation of swine wastewater has remained an effective way to increase biogas yields (Møller et al, 2000). Pre-treatment of slurry by the separation to produce a highly-concentrated solid fraction could significantly increase the biogas potential per waste volume in the pre-treatment to produce a highly-concentrated solid fraction and biogas potential (Møller et al., 2002; Møller et al., 2004). Deng et al. (2012) realized a good separation outcome of a chemical oxygen demand (COD) of 9661 mg L-1 via precipitation and sedimentation to separate raw swine slurry.
Solid-liquid separation of swine wastewater could reduce COD, BOD5, SS, TS, TP, and TKN by 10.03%, 19.23%, 10.18%, 6.90%, 14.42%, and 14.14%, respectively (Yang et al, 2015). The swine manure treatment gave high TS and COD removal efficiency equivalent to an average of 71.4% and 96.8%, respectively, when solid-liquid separation using screw pressing followed by coagulation-flocculation and nitrification and denitrification of the liquid fraction was applied (Riano and Garcia-Gonzalez, 2014).
Kunz et al. (2009) reported that the solid-liquid separation process must be done early enough to avoid the extensive degradation of swine manure and excessive solubilization of mineral and organic nutrients under tropical climatic conditions. Finally, the first strategy that must be done is solid-liquid separation to avoid particulate materials from exceeding the further chemical or biological treatment in advance of swine wastewater treatment processes.
Table 1 Comparison of conventional methods of swine wastewater treatment with their corresponding nutrient removal efficiencies.
|
Conventional method of treating swine wastewater |
COD(%) |
BOD(%) |
N(%) |
P(%) |
TAN(%) |
References |
|
Solid-liquid separation |
10.03 96.8 |
19.23 N/A |
14.14 N/A |
14.42 N/A |
N/A N/A |
Yang et al., 2015 Riano and Garcia-Gonzalez, 2014 |
|
Chemical treatment |
85.1
N/A N/A N/A 83 |
N/A
N/A N/A N/A N/A |
75.0
N/A N/A N/A 90 |
N/A
>97.0 N/A >99 97 |
N/A
91 >94 >90 N/A |
Lim and Kim, 2015; Lim et al., 2016 Huang et al., 2015 Huang et al., 2017 Huang et al., 2016 Ryu and Lee, 2010 |
|
Constructed wetlands
|
30-50 83.4 |
N/A N/A |
37-51 84 |
13-26 90.4 |
N/A N/A |
Poach et al., 2014 Xiam et al., 2010 |
|
Anaerobic Digestion
|
95 95.5 96.98 N/A
76 |
N/A 99.6 N/A N/A
N/A |
93 94.3 91.69 43.1-64.3
80 |
N/A N/A 74.71 96-99
N/A |
99 99.4 98.95 96
96 |
Sui et al., 2018 Deng et al., 2006 Yang et al., 2016 Molinuevo-salco et al., 2016 Daverey et al., 2013 |
Note: COD: chemical oxygen demand; BOD: biological oxygen demand; N: nitrogen; P: phosphorus; TAN: total ammonium nitrogen; N/A: none applicable
Chemical treatment processes of swine wastewater
Swine wastewater can be effectively treated by the application of chemical treatment methods in tandem with physical and biological methods. High ammonia concentration is mostly present in swine wastewater along with high-level organics. Particularly, ammonia favors the reduction of dissolved oxygen (DO), eutrophication, and red tidal occurrence in water courses, which has been a major concern (Havlikoba et al. 2008; Shin et al. 2008). Swine wastewater must be properly treated in accordance with the law standards before being released into the environment to avoid the cleaving aquatic system and the adjacent waters.
Currently, ammonia stripping, struvite precipitation, and biological methods are mostly used for the removal and recovery of nitrogen (N) and phosphorus (P) in wastewater (Xing et al. 2016; Sun and Sun, 2012; Tao and Ukwuani, 2015; Huang et al. 2015; Zhang et al. 2016). Biological processes are recognized as the utmost cost-effective method to treat swine wastewater (Cooper et al. 1994). Significantly, biological processes are affected by high concentration of ammoniacal nitrogen in swine wastewater, which can inhibit the activity of microorganisms (Valdivelu et al. 2006). Hence, before biological treatment is instigated, N and P nutrients in swine wastewater need to be pretreated in physiochemical processes such as ammonia stripping and struvite precipitation. Struvite precipitation is a favorable method for the concurrent recovery of phosphate (PO4-P) and total ammonia nitrogen (TAN, NH3, and NH4+) (Huang et al. 2016), and the subsequent struvite can be utilized as a slow-release fertilizer in agriculture. However, there is a need to supplement some Mg2+ to the wastewater for the concurrent recovery of TAN and PO4-P by struvite precipitation since swine wastewater lacks Mg2+. Among various Mg sources, such as pure MgCl2 and MgSO4 salt (Li et al. 1999), seawater (Kumashiro et al. 2001), magnesite (Gunay et al. 2008), bittern (Lee et al. 2003), and brucite (Huang et al. 2012), MgO (Huang et al. 2015) is the most promising Mg2+ supplement due to its low cost.
In most studies, the recovery and removal of nutrients from swine wastewater by employing a struvite recycling process mostly used magnesium and phosphate as the active supplement to the effluent. Using the magnesium and potassium (MP) as sources of struvite precipitation achieved a removal efficiency of the TAN close to that of using pure chemicals (MgCl2·6H2O and Na2HPO4·12H2O) (Huang et al 2016). According to Huang et al (2016), pyrolyzing the mixture of struvite and brucite at Mg (OH)2: NH4+ (1:1), and the ammonium released efficiency achieved 89% by 150 ℃ for 3 h. In addition, Huang et al. (2017) used three types of magnesium sources for nutrient recovery from swine wastewater: Mg alloy, Low-grade magnesium oxide (LG-MgO), Mg-containing supernatant followed by the removal of TAN by ammonia stripping using LG MgO (Huang et al. 2017). The PO4-P in swine wastewater was recovered as struvite with high purity through chemical corrosion of Mg alloy, however, a very low-purity struvite was achieved using LG-MgO as the Mg source of struvite precipitation (Huang et al. 2017). Almost >94% of TAN were removed from the effluent produced from the phosphate-retrieval stage, when ammonia stripping at an aeration rate of 5L air/l wastewater· min, LG-MgO dosage of 8 g/l, and a temperature of 25°C for 180 min. The optimal Mg source for the recovery of phosphate was Mg-containing supernatant (Huang et al. 2017).
Huang et al. (2016) investigated the feasibility of the simultaneous recovery of total phosphorus (TP) and removal of TAN from swine wastewater by a coupled electrochemical process. The result showed that phosphate recovery efficiency was 99% at a current density of 2mA/cm2 for 45 min, when the magnesium alloy was electrolyzed as magnesium source for struvite crystallization. Also, efficiency of > 90% of ammonium nitrogen removal from swine wastewater was achieved by recycling the electrolyzed product of struvite using seawater as a supporting solution. The use of chemical treatment method provides a feasible way to improve pollutant removal efficiencies from swine wastewater for further biological treatment processes.
Constructed wetlands
Constructed wetlands (CWs) have been identified as a sustainable wastewater management option, which has been successfully used for treating various wastewaters worldwide. Constructed wetlands (CWs) are engineered systems designed and constructed to utilize the natural functions of wetland vegetation, soil media, and their associated microbial associated assemblages for wastewater treatment within a more controlled environment (Kadlec and Knight, 1996). CWs have been recognized as a sustainable wastewater management option due to their high pollutant removal efficiency, easy operation and maintenance, low cost, good potential of water and nutrient reuse, tolerance to high variability, and function as significant wildlife habitat (Mitsch and Gosselink, 2000; Kadlec and Wallace, 2008).
Continuous discharge of swine wastewaters from lagoon to agricultural lands is no doubt a risk to the surface and groundwater quality. Wastewater from land-based animal farm introduces pathogens, antibiotics, and heavy metals into the fields, thus consequently contaminate local soil and groundwater (Ma et al., 2013; Brooks et al., 2014; Kessleret al., 2014; Tao et al., 2014; Adhikari et al., 2015). Meers et al. (2008) and Chen et al. (2008) reported that CWs is a good alternative treatment system that can be used for treating swine wastewater to reach the discharge standard.
Poach et al. (2004) investigate the ability of marsh-pond-marsh (m-p-m) CWs to treat wastewater from a confined swine operation over varying nitrogen loads during two experimental periods, summer, and winter. According to Poach et al (2004), the efficiencies of each m-p-m constructed wetland system removed TSS, COD, N, and P from swine wastewater by 35%–51%, 30%–50%, 37%–51%, and 13%–26% respectively, and total N treatment efficiencies were significantly lower during the winter experimental period compared to the summer due to air temperature decrease in winter. Stone et al. (2004) used existing m-p-m CWs design to analyze swine lagoon wastewater treatment and reported that the CWs were effective in N treatment of swine lagoon wastewater in which the mean TN and ammonia-N concentration removal rate reached approximately 30%, whereas for the P treatment it was not as effective for being 8% only.
In order to select a suitable phosphorus removal substrate for CWs treating swine wastewater, Wang et al. (2013) found that oyster shell was appropriate for phosphorus removal in CWs besides broken bricks and zeolite. Significant phosphorus removal was attained in model tests, in which an oyster shell substrate was used in vertical-flow wetlands treating swine wastewater from an anaerobic tank. The results additional recommended that phosphorus removal could be enhanced by increasing the HRT from 0.566 d to 1.649 d (Wang et al. 2013). Luo et al. (2017) investigated the phosphorus removal efficiency from lagoon-pretreated swine wastewater was 70.1%–89.4% of pilot-scale surface flow constructed wetlands planted with Myriophyllum aquaticum.
In a high-strength treatment of swine wastewater, Peng et al. (2012) tested a novel circular-flow corridor wetland; and the system showed a good performance with high efficient removal rates of COD, NH4-N, and TP in average removal efficiency of above 93.9%. Wang et al. (2014) compared a study of subsurface vertical flow constructed wetland (VSSF) at four shunt ratios to treat swine wastewater when the hydraulic loading rate (HLR) was 0.02 m3. (m2d)-1, where the VSSF was effective in removal of TSS, TP, COD, and BOD, and the shunt ratio had no significant influence on the pollutant except for N. The optimal shunt ratio was 1:2, as VSSF had the best effect on swine wastewater treatment, especially for TN (Wang et al. 2014).
Three varieties of Italian ryegrass, Dryan, Tachimasari, and Waseyutaka were used to remove nutrients and antibiotics from swine wastewater in a constructed macrophyte floating bed system, obtaining good reduction in the levels of nutrients and COD. It was also found that for Dryan, total N was reduced by 84.0%, total P by 90.4%, COD by 83.4%, and sulfonamide antimicrobials by 91.8%–99.5%. Constructed wetlands (CWs) appear as a cost-effective treatment, since they can remove a broad range of contaminants by a combination of physical, chemical, and biological processes at a low cost.
Anaerobic digestion
Anaerobic digestion systems characterize a feasible technology for tropical and subtropical countries where the weather permits the operation of reactors at air temperature. These robust systems need low investment, low costs, and have recently been enhanced to eliminate organic matter and the production of methane gas, decrease the sludge output, and demand for smaller areas (Shin et al., 2011). Anaerobic digestion is an efficient alternative technology for the treatment of wastewater, agricultural waste, food processing waste, and fruit and vegetable debris as well as for sludge stabilization. Its benefits are the production of renewable fuel as biogas, the agricultural recycling of organic matter, and the recovery of the remaining nutrients in the effluents of the anaerobic reactors (Appels et al., 2011). Anaerobic digestion is an important alternative to land application in swine waste treatment, because it reduces pollution and recovers methane.
Co-digestion has been defined as the anaerobic treatment of a mixture of at least two different substrates to improve the efficiency of the anaerobic digestion process (Álvarez et al., 2010). Co-digestion of manures and other substrates could overcome challenges associated with anaerobic digestion of agro-residues, such as low pH of the substrate, poor buffering capacity, and the possibility of the high volatile fatty acid (VFA) (Banks and Humphreys, 1998; Campos et al., 1999). Mazareli et al. (2016) studied anaerobic co-digestion of vegetable waste and swine wastewater in high-rate horizontal reactors with fixed bed, and the maximum methane production of 1.08 L CH4 (L d)-1 with 70% of total COD removed and converted into methane by using 70% swine wastewater and 30% vegetable wastewater. Di Maria et al. (2015) reported that fruit and vegetable wastes are promising substrates for co-digestion processes, because of the high production of biogas production and the stabilization of the reactor.
Wen et al. (2016) used two strains of photosynthetic bacteria, Rhodobacter blasticus and Rhodobacter capsulatus to test the treatment of anaerobical digestion of swine wastewater. The result show that anaerobically digested swine wastewater could be treated effectively by photosynthetic bacteria. On the condition of 1:1 mixture proportion of two bacteria, 10.0% (v/v) inoculation size, initial pH at 7.0, and initial COD of 4800 mg L-1, the COD removal rates increased to 83.3%, which is 19.3% and 10.6% higher than that by Rhodobacter blasticus and Rhodobacter capsulatus respectively, the corresponding biomass was 852.3 mg L-1 (Wen et al., 2016). Revalorization of microalgae biomass through anaerobic digestion of swine wastewater achieved methane yields in the range of 106–146 and 171 mL CH4 g COD-1 for biomasses grown in batch and semi-continuous modes, respectively (Molinuevo-Salces et al., 2016). Perazzoli et al. (2016) quantified biomethane from anaerobic degradation of microalgae biomass that was harvested from a field-scale tank reactor simulating phycoremediation of swine wastewater. The results show a high biomethane yield of 103.5 LN CH4 (kg biomass)-1 vs 44 LN CH4 (kg biomass)-1. The results propose that biomethane production in digesters was enhanced by incorporating microalgae biomass harvested from algae-based swine wastewater digestate treatment (Perazzoli et al., 2016). Deng et al. (2016) also reported that a combined process of liquid swine manure (LSM) pretreatment and microalgae cultivation in a thermophilic AD was an effective way to utilize nutrients in LSM to yield efficient valuable microalga products.
Using microalgae to treat swine wastewater
Microalgae have attracted increasing attention due to its prospect to aid as feedstock for biofuels and wastewater bioremediation. Cultivation of microalgae with wastewater is regarded as an ideal situation for attaining microalga feedstock for biofuel use. Municipal wastewater has been regularly used for microalgae cultivation, but swine wastewater is rich in nitrogen nutrients. Swine wastewater contains nitrogen, carbon, and phosphate, which is an important source of nutrients for microalga growth. The use of microalgae for swine wastewater treatment has thus been extensively studied in the past, and the results of some pilot-scale setups having been published (Table 2) (Godos et al., 2009; Kao et al., 2012). Wastewater treatment systems using microalgae have benefits of low energy requirements, minor sludge formation, and a decline of greenhouse emissions, and productive use of wastewater as compared with conventional activated sludge and/or anaerobic treatment methods of swine wastewater (Cheng et al., 2019).
Microalgae Cultivation modes
The key microalgae cultivation modes in wastewaters are photoautotrophic, heterotrophic, and mixotrophic cultivations (Chew et al., 2018). Due to large amount of organic carbon in swine wastewater, it makes it necessary for microalgae to be cultivated in mixotrophic and heterotrophic cultivation modes (Wang et al., 2015).
Table 2 Comparison of different microalgae cultivation systems and corresponding specifications
|
Cultivation system |
Microalgae |
COD (%) |
NH4-N (%) |
N (%) |
P (%) |
Biomass Productivity |
Lipid content/productivity |
Notes |
References |
|
Open pond |
Botryococcus braunii |
N/A |
N/A |
40.8 |
93.3 |
0.94 mg/L |
23.8% |
In aerated swine lagoon |
Liu et al., 2013 |
|
88±6 |
N/A |
76±11 |
N/A |
21-28 g/m2d |
N/A |
In HRAP |
Godos et al., 2009 |
||
|
Chlorella sp., Scenedesmus sp |
N/A |
N/A |
N/A |
N/A |
0.024 g/L/d |
N/A |
Raceway pond |
Nwoba et., 2006 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Closed system |
Chlorella vulgaris JSC-6 |
60-70 |
40-90 |
N/A |
N/A |
3.96 g/L |
N/A |
Both in mixotrophic and heterotrophic |
Wang et al., 2015 |
|
Neochloris aquatic CL-M1 |
N/A |
N/A |
62 |
28 |
0.56±0.35 g dwt/L |
0.31±0.03g/L & 33%±3% |
|
Abou-Shanab etal., 2013 |
|
|
Chlorella vulgaris |
58.39 |
N/A |
90.51 |
90.54 |
N/A |
N/A |
Undiluted swine wastewater |
Wen et al., 2017 |
|
|
72.91 |
80.50 |
N/A |
96.90 |
N/A |
N/A |
Combination of absorption stripping & acidification |
Cao et al., 2018 |
||
|
Scenedesmus obliquus |
75.29 |
N/A |
74.63 |
81.73 |
0.311 g/L/d |
N/A |
Photobioreactor (PBR) bag |
Xu et al., 2015 |
|
|
Chlorella zofingiensis |
N/A |
N/A |
82.7 |
98.17 |
0.296 g/L/d |
N/A |
Bubble column PBR |
Zhu et al., 2013 |
|
|
Chlorella vulgaris (UTEX 2714) |
30.2-42.2 |
98.3-99.3 |
46.9-71.4 |
30.2-80.4 |
132.2-440.3 mg/L/d |
N/A |
Mixture of swine waste and centrate |
Den et al., 2018 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Biofilm attached system |
Botryococcus braunii |
N/A |
N/A |
N/A |
N/A |
5.5 g/m2/d |
2.34g/m2/d |
Biofilm attached system |
Cheng et al., 2013 |
|
Botryococcus braunii SAG 807-1 |
N/A |
N/A |
N/A |
N/A |
4.78 g/m2/d |
2.52g/m2/d |
Cheng et al., 2017 |
||
|
Chlorella pyrenoidosa |
74.8 |
75.9 |
N/A |
68.4 |
5.03 g/m2/d |
35% |
Cheng et al., 2017 |
Photoautotrophic cultivation
Photoautotrophic cultivation is a basic method in low energy consuming strategy for cultivating microalgae, and typically developed in two cultivating systems known as open pond system and closed photobioreactor system (SundarRajan et al., 2019). In photoautotrophic cultivation mode, microalgae need light (natural or artificial) to grow and produce new biomass. Photoautotrophic cultivation is the most widely used microalgae cultivation mode. The lipid content of microalgae under photoautotrophic cultivation has a large disparity in the type of microalgae species, which range from 5% to 68%. Usually a nitrogen-limiting or nutrient limiting condition was used to raise the lipid productivity in microalgae (Mata et al., 2010). Consequently, attaining higher lipid content is typically at the expense of lower biomass productivity. Hence, lipid productivity is a key factor for potential industrial application of microalga cultivation with regard to oil production. As reported in the literature, lipid production can reach maximum of 179 mg·L-1·d-1 under photoautotrophic cultivation of Chlorella sp. (Chiu et al., 2008). The key benefit of using autotrophic cultivation to produce microalga oil is the intake of CO2 as a carbon source and low-cost medium for the cell growth and oil production.
Heterotrophic cultivation
In heterotrophic cultivation, microalgae are grown without light and are fed with a carbon source (such as sugar) to produce new biomass. Certain microalgae species can develop under photoautotrophic environmentsand use organic carbon under dark conditions. The advantage is that heterotrophic microalgae can grow rapidly without light to reach high biomass concentrations. Heterotrophic cultivation is more economical and easier to set-up than photoautotrophic mode since the cultivation situations are simple. This makes heterotrophic cultivation mode possible for different industrial processes such as biofuel production and wastewater treatment to be done simultaneously (Ugwu et al., 2008; Perez-Garcia et al. 2011). Many microalgae species, such as Chlorella protothecoides, show higher lipid content by up to 40% during heterotrophic growth better than phototrophic conditions (Xu et al., 2006). In both open ponds and closed photobioreactors, many studies have confirmed the benefits of heterotrophic cultivation as compared to photoautotrophic conditions.
Mixotrophic cultivation
Mixotrophic cultivation harnesses both photoautotrophic and heterotrophic capacities of microalgae, where CO2 and organic carbon are assimilated in cells simultaneously. Outcomes of mixotrophic microalgae cultivation modes as compared with the heterotrophic cultivation condition, shows more biomass productivities, accumulation of lipids and carbohydrates (Chew et al., 2018; Wang et al., 2015). The light limitation exhibited in pure microalgae photoautotrophic conditions could overcome through mixotrophic cultivation (Ceron Garcia et al., 2006). Chojnacka and Noworyta (2004) investigated the growth of Spirulina sp. in photoautotrophic, heterotrophic, and mixotrophic cultures, the highest growth rate was attained under a mixotrophic conditions level of light intensity > 30 w·m−2 and glucose concentrations > 0.5 g·L−1. Hu et al. (2012) used mixotrophic cultivation of Chlorella sp. with 0.1 (v/v) propionic, butyric, and acetic acids each to treat swine wastewater, and found that acidogenically digested manure supported algal growth, improved nutrient removal, and enhanced total lipid yields in 7-day batch cultivation. Direct application of microalgae mixotrophic mode in wastewater might provide a simplified way to simultaneously produce algal oil and treat high organic content of wastewater.
Microalgae culture systems for swine wastewater treatment
Cultivation in suspension systems is used typically for microalga growth in swine wastewaters for bioremediation, and bio-products production includes closed and open bioreactors. Also, biofilm attached cultivation was employed to improve microalga cultivation modes with higher biomass productivity and was more economically viable (Cheng et al., 2017). The choice of a suitable cultivation system is critical for growing microalgae, and can be determined by the microalgae species in question, the cost of culturing system, the final product, the source of CO2 and the nutrients needed (Razzak et al. 2013).
Open systems
Microalgae cultivation in open ponds has been used for large-scale production because of its simple construction, relatively easy operation, and low operation costs (Fig.1). However, open pond systems rely heavily on the natural environment of the pond site, considerably as the temperature rise loss of water by evaporation increases, and pollution is likely to occur. Recently, types of open pond system in use consist of raceway pond, circular ponds, slope system, and tanks and these have been established for many years. High rate algal ponds under optimal conditions supported with TKN and COD removal efficiencies of 88%± 6% and 76%±11%, respectively, and microalga biomass yields of 22 to 28 g/m-2d-1 for the treatment of swine wastewater (Godos et al. 2009). Liu et al. (2013) reported that Botryococcus braunii could successfully remove 93.3% and 40.8% of the TP and TN, respectively, with algal biofuel content of 23.8% in aerated swine lagoon wastewater cultivation. Phytoremediation was investigated to treat swine manure lagoon wastewater and the results reveal that N and P removal efficiency could be improved by using a metagenomic approach (Ye et al., 2016).

Fig.1 Open cultivation technique: (a) Paddle wheel raceway pond. (b) Circular pond (SundarRajan et al., 2019).
Closed systems
Closed photobioreactors are introduced to overcome challenges that related to open systems (Fig.2). Generally, they are more efficient in cell growth with better control of the culture conditions and growth parameters, such as pH, temperature, mixing, CO2, and O2 concentrations (Posten, 2009). However, the closed system has its limitation in terms of difficulties in scale-up, over-heating, requiring higher capital and operating cost (Posten and Schaub, 2009). Nutrient removal efficiencies and biomass yield can be enhanced by efficient culture procedures. The most frequently used closed PBRs consist of flat plate reactors, bubble-column reactors, and tubular reactors (Posten, 2009; Posten and Schaub, 2009; Ugwu et al. 2008). Chlorella vulgaris JSC-6 was used in treating swine wastewater in closed photobioreactors, and the removal efficiency of COD and NH3-N were nearly 60%–70% and 40%–90%, in both mixotrophic and heterotrophic culture, respectively (Wang et al. 2015). Chlorella znofingeiensis cultured in autoclaved swine wastewater and pollutants were efficiently reduced in tubular bubble column PBR (Zhu et al. 2013). Additionally, Neochloris aquatica CL-M1 was adapted to grow in swine wastewater and the results show that the utmost removal efficiency of COD and NH3-N were 81.7% and 96.2%, respectively (Wang et al. 2017). It was found that microalgae in swine wastewater seemed increased in biomass concentration, thus removed pollutants. According to Wang et al. (2015), the growth of Chlorella vulgaris JSC-6 reached the highest biomass production of 3.96 g/L. Also, the highest biomass concentration and carbohydrate content reached 6.10 g/L and 50.46%, respectively, when N/P ratio was 5/1 (Wang et al., 2015). Indigenous microalga strain Chlorella vulgaris that was isolated from swine wastewater effluent, the removal efficiencies of TN and TP were 90.51% and 91.54%, respectively (Wen et al., 2017). Deng et al., (2018) cultivated Chlorella vulgaris UTEX 2714 in a mixed wastewater (swine waste and centrate) and the nutrient removal efficiencies could reach 99.3%, 71.4%, 42.2%, and 80.4% for NH4+-N, TN, COD, and TP, respectively, with a biomass productivity of 132.2–440.3 mg/L/d. Biomass of Chlorella vulgaris (UTEX 2714) that developed on the mixed wastewater could be used as a good value feedstock for bioenergy production. Closed reactors are appropriate for photoautotrophic, mixotrophic, or heterotrophic algae cultivation.
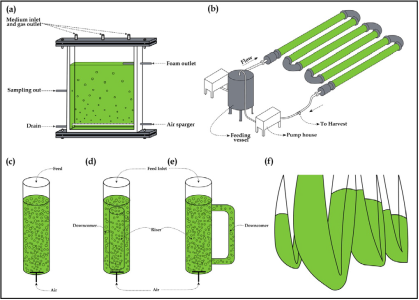
Fig.2 Closed cultivation technique.(a) Flat-panel PBR; (b) Horizontal tubular PBR; (c)Bubble column PBR; (d) Internal loop Airlift PBR; (e) External loop Airlift PBR; and (f) Largescale plastic bag PBRs (SundarRajan et al., 2019)
Biofilm attached cultivation
In biofilm attached cultivation, biofilm is the basic character (Fig.3). To solve the problem of microalgae biomass yield for industrial application, the biofilm-attached cultivation is a substitute pathway. The present limitations of the open pond and closed systems such as cost, high-energy consumption, and time-consuming can be overcome by application of algal biofilm reactors (He and Xue, 2010; Riuz-Marin et al., 2010). According to Hoh et al. (2016) algal biofilm biomass productivity could reach 2.8 times higher than that of the suspended system. Numerous kinds of biofilm reactors have been developed, including horizontal, vertical, flow cell, rotating, and rocker bioreactors.
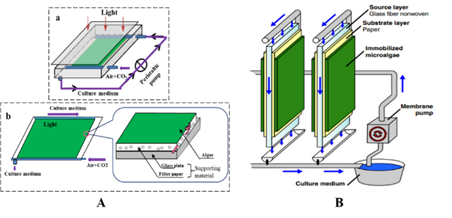
Fig.3 The schematic diagrams of attached cultivation devices. (A) Attached cultivation module of the photobioreactor, the residual medium was recycling. The medium was propelled to the system by a peristaltic pump when it flowed through the chamber during the cultivation. (B) The detailed structure of the cultivation surface of the attached photobioreactor. Permission to use and adapted from Cheng et al. (2014) and Cheng et al. (2017)
Biofilm attached microalgae cells on a vertical artificial surface and recorded biomass productivity of 50–80 g·m-2·d-1 for Scenedesmus obliguus in an outdoor, with total solar radiation of 5.2%–8.3% (Liu et al., 2013). Similar high biomass productivities of 6.5 g·m-2·d-1 was obtained at the initial stage cultivation of Botryococcus braunii in a single layer attached method (Cheng et al. 2013). In addition, comparing the biofilm attached culture with aqueous-suspension cultivation, the Botryococcus braunii biomass productivities were 4.78 g·m-2·d-1 and 4.43 g·m-2·d-1, respectively (Cheng et al. 2017a). Moreover, the algae Chlorella pyrenoidosa was cultivated in unsterilized wastewater by biofilm attached method, the biomass yield, lipid productivity and lipid content could reach of 5.03 g·m-2·d-1, 1.80 g·m-2·d-1, and 35.9%, respectively (Cheng et al. 2017b).
In addition, we studied the removals of COD, TN, and TP by algal biofilm-attached cultivation in swine wastewater. Accordingly, effluent concentration of 85.60 mg·L-1, 35.22 mg·L-1, 44.64 mg·L-1, and 1.13 mg·L-1 for COD, NH4+-N, TN, and TP, the nutrient removal efficiencies were of 98.2%, 95.7%, 95.6% and 96.2%, respectively in a sequencing batch biofilm reactor (Hai et al. 2015). In other related studies, nitrogen and phosphorus removal efficiencies could be 18% and above 33%, respectively in a single biofilm sequencing batch reactor (Ra and Lau 2010). The algae biofilm attached cultivation signifies a favorable technology for economically sustainable production of microalgae biofuels and nutrient removal efficiencies from swine wastewater.
Conclusion
This review describes the various perspective ways of swine wastewater treatment and discusses their merits and limitations. Although various conventional ways have been used in treating swine wastewater as described in literature, they are mostly employed to aid in further biological advance swine wastewater treatment processes. The used of microalgae appear to be a successful treatment for nutrient-rich swine wastewater. Present microalgae wastewater treatment and bioproduct production, mostly depend on suspended algal culture and it has been limited by low productivity of algal biomass, high operating cost, and high energy consumption in harvesting algal cells. Biofilm attached cultivation methods were developed to overcome recent limitations in swine wastewater treatment. Microalgae biomass productivity with biofilm-attached method could reach 2.8 times higher than that of the suspended system for bioproduct production. Large-scale application of using microalgae to treat swine wastewater requires further research in terms of engineering and optimization of culturing conditions for efficient wastewater treatment and high algal biomass productivity.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (No. 31560724), Natural Science Foundation of Jiangxi Province (No. 20171BAB214014), China Postdoctoral Science Foundation (No. 2016M600616, 2017T100583), and Key Laboratory of Poyang Lake Ecological Environment and Resource Development (No. PK2017001).
References
Abou-Shanab, R. A. I., Ji, M.-K., et al. (2013). Microalgal species growing on piggery wastewater as a valuable candidate for nutrient removal and biodiesel production. Journal of Environmental Management, 115, 257-264. doi: https://doi.org/10.1016/j.jenvman.2012.11.022
Álvarez, J., Otero, L., Lema, J. (2010). A methodology for optimising feed composition for anaerobic co-digestion of agro-industrial wastes. Bioresource Technology, 101(4):1153-1158.
Ayre, JM., Moheimani, NR., Borowitzka, MA. (2017). Growth of microalgae on undiluted anaerobic digestate of piggery effluent with high ammonium concentrations. Algal Research-Biomass Biofuels and Bioproducts, 24:218-226.
Banks, C., Humphreys, P. (1998). The anaerobic treatment of a ligno-cellulosic substrate offering little natural pH buffering capacity. Water Science and Technology, 38(4):29-35.
Brooks, J., Adeli, A., McLaughlin, M. (2014). Microbial ecology, bacterial pathogens, and antibiotic resistant genes in swine manure wastewater as influenced by three swine management systems. Water Research, 57:96-103.
Cai, T., Park, SY., Li, Y. (2013). Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renewable & Sustainable Energy Reviews, 19:360-369.
Cao, L., Zhou, T., Li, Z., et al. (2018). Effect of combining adsorption-stripping treatment with acidification on the growth of Chlorella vulgaris and nutrient removal from swine wastewater. Bioresource Technology, 263, 10-16. doi: https://doi.org/10.1016/j.biortech.2018.04.094
Ceron Garcia, M., Camacho, F., Miron, A., et al. (2006). Mixotrophic production of marine microalga Phaeodactylum tricornutum on various carbon sources. microbiology and Biotechnology, 16(5):689-694.
Cheng, D. L., Ngo, H. H., Guo, W. S., et al. (2019). Microalgae biomass from swine wastewater and its conversion to bioenergy. Bioresource Technology, 275, 109-122. doi: https://doi.org/10.1016/j.biortech.2018.12.019
Cheng, P., Ji, B., Gao, L., et al. (2013). The growth, lipid and hydrocarbon production of Botryococcus braunii with attached cultivation. Bioresource Technology, 138:95-100.
Cheng, P., Wang, Y., Liu, T., et al. (2017). Biofilm attached cultivation of Chlorella pyrenoidosais a developed system for swine wastewater treatment and lipid production. Frontiers in Plant Science, 8.
Cheng, P., Wang, Y., Yang, Q., et al. (2017). Comparison of growth, hydrocarbon accumulation and metabolites of Botryococcusbraunii between attached cultivation and aqueous-suspension cultivation. International Journal of Agricultural and Biological Engineering, 10(1):134-141.
Chew, K.W., Chia, S.R., Show, P.L., et al. (2018). Effects of water culture medium, cultivation systems and growth modes for microalgae cultivation: a review. J. Taiwan Inst. Chem. Eng. 91:332–344.
Chiu, S., Kao, C., Chen, C., et al. (2008). Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresource Technology, 99(9):3389-3396.
Chojnacka, K., Noworyta, A. (2004). Evaluation of Spirulina sp. growth in photoautotrophic, heterotrophic and mixotrophic cultures. Enzyme and Microbial Technology, 34(5):461-465.
Cooper, P., Day, M., Thomas, V. (1994). Process Options for Phosphorus and Nitrogen Removal from Wastewater. Water and Environment Journal, 8(1):84-92.
Deng, L., Chen, Z., Yang, H., et al. (2012). Biogas fermentation of swine slurry based on the separation of concentrated liquid and low content liquid. Biomass and Bioenergy, 45:187-194.
Deng, X., Gao, K., Zhang, R., et al. (2017). Growing Chlorella vulgaris on thermophilic anaerobic digestion swine manure for nutrient removal and biomass production. Bioresource Technology, 243:417-425.
Deng, X., Gao, K., Addy, M., et al. (2018) Growing Chlorella vulgaris on mixed wastewaters for biodiesel feedstock production and nutrient removal. J. Chem. Technol. Biotechnol. 93 (9):2748–2757.
DiMaria, F., Sordi, A., Cirulli, G., et al. (2015). Amount of energy recoverable from an existing sludge digester with the co-digestion with fruit and vegetable waste at reduced retention time. Applied Energy, 150:9-14.
García, D., Posadas, E., Blanco, S., et al. (2018). Evaluation of the dynamics of microalgae population structure and process performance during piggery wastewater treatment in algal-bacterial photobioreactors. Bioresource Technology, 248:120-126.
Godos, I., Blanco, S., García-Encina, P., et al. (2009). Long-term operation of high rate algal ponds for the bioremediation of piggery wastewaters at high loading rates. Bioresource Technology, 100(19):4332-4339.
Gunay, A., Karadag, D., Tosun, I., et al. (2008). Use of magnesit as a magnesium source for ammonium removal from leachate. Journal of Hazardous Materials, 156(1):619-623.
Hai, R., He, Y., Wang, X., et al. (2015). Simultaneous removal of nitrogen and phosphorus from swine wastewater in a sequencing batch biofilm reactor. Chinese Journal of Chemical Engineering, 23(1):303-308.
Havlikova, M., Kroeze, C., Huijbregts, MAJ. (2008). Environmental and health impact by dairy cattle livestock and manure management in the Czech Republic. Science of the Total Environment, 396(2):121-131.
He, S., Xue, G. (2010). Algal-based immobilization process to treat the effluent from a secondary wastewater treatment plant (WWTP). Journal of Hazardous Materials, 178(1):895-899.
Hoh, D., Watson, S., Kan, E. (2016). Algal biofilm reactors for integrated wastewater treatment and biofuel production: A review. Chemical Engineering Journal, 287:466-473.
Hu, B., Min, M., Zhou, W., et al. (2012). Enhanced mixotrophic growth of microalga Chlorella sp. on pretreated swine manure for simultaneous biofuel feedstock production and nutrient removal. Bioresource Technology, 126:71-79.
Huang, H., Guo, G., Zhang, P., et al. (2017a). Feasibility of physicochemical recovery of nutrients from swine wastewater: Evaluation of three kinds of magnesium sources. Journal of the Taiwan Institute of Chemical Engineers, 70:209-218.
Huang, H., Liu, J., Wang, S., et al. (2016c). Nutrients removal from swine wastewater by struvite precipitation recycling technology with the use of Mg3(PO4)2 as active component. Ecological Engineering, 92:111-118.
Huang, H., Song, Q., Wang, W., et al. (2012). Treatment of anaerobic digester effluents of nylon wastewater through chemical precipitation and a sequencing batch reactor process. Journal of Environmental Management, 101:68-74.
Huang, H., Xiao, D., Liu, J., et al. (2015). Recovery and removal of nutrients from swine wastewater by using a novel integrated reactor for struvite decomposition and recycling. Scientific Reports, 5:10183.
Huang, H., Zhang, D., Li, J., et al. (2017b). Phosphate recovery from swine wastewater using plant ash in chemical crystallization. Journal of Cleaner Production, 168:338-345.
Huang, H., Zhang, P., Zhang, Z., et al. (2016b). Simultaneous removal of ammonia nitrogen and recovery of phosphate from swine wastewater by struvite electrochemical precipitation and recycling technology. Journal of Cleaner Production, 127:302-310.
Huang, HM., Liu, J., Xiao, J., et al. (2016a). Highly efficient recovery of ammonium nitrogen from coking wastewater by coupling struvite precipitation and microwave radiation. Chemical Engineering Journal, 436:88-96.
Kadlec, RH., Knight, RL. (1996). Treatment wetlands. CRC Press Inc., Boca Raton, FL.
Kadlec, RH., Wallace, S. (2008). Treatment wetlands. CRC Press Inc., Boca Raton, FL., Second edition.
Kadir, W.N.A., Lam, M.K., Uemura, Y., et al. (2018). Harvesting and pretreatment
of microalgae cultivated in wastewater for biodiesel production: a review. Energy Convers. Manage. 171:1416–1429.
Kao, C., Chiu, S., Huang, T., et al. (2012). Ability of a mutant strain of the microalga Chlorella sp. to capture carbon dioxide for biogas upgrading. Applied Energy, 93:176-183.
Kothari, R., Pathak, VV., Kumar, V., et al. (2012). Experimental study for growth potential of unicellular alga Chlorella pyrenoidosa on dairy waste water: An integrated approach for treatment and biofuel production. Bioresource Technology, 116:466-470.
Kumashiro, K., Ishiwatari, H., Nawamura, Y. (2001). A pilot plant study on using seawater as a magnesium source for struvite precipitation. In: Proceedings of the second international conference on the recovery of phosphorus from sewage and animal wastes. Noordwijkerhout, The Netherlands.
Kunz, A., Steinmetz, R.L.R., Ramme, MA., et al. (2009). Effect of storage time on swine manure solid separation efficiency by screening. Bioresource Technology, 100(5):1815-1818.
Lee, SI., Weon, SY., Lee, CW., et al. (2003). Removal of nitrogen and phosphate from wastewater by addition of bittern. Chemosphere, 51(4):265-271.
Li, X.Z., Zhao, Q.L., Hao, XD. (1999). Ammonium removal from landfill leachate by chemical precipitation. Waste Management, 19(6):409-415.
Li, Y., Chen, YF., Chen, P., et al. (2011). Characterization of a microalga Chlorella sp well adapted to highly concentrated municipal wastewater for nutrient removal and biodiesel production. Bioresource Technology, 102(8):5138-5144.
Liu, J., Ge, Y., Cheng, H., et al. (2013). Aerated swine lagoon wastewater: A promising alternative medium for Botryococcus braunii cultivation in open system. Bioresource Technology, 139:190-194.
Liu, J., Ge, Y., Cheng, H., et al. (2013a). Aerated swine lagoon wastewater: A promising alternative medium for Botryococcus braunii cultivation in open system. Bioresource Technology, 139:190-194.
Liu, T., Wang, J., Hu, Q., et al. (2013). Attached cultivation technology of microalgae for efficient biomass feedstock production. Bioresource Technology, 127:216-222.
Luo, P., Liu, F., Liu, X., et al. (2017). Phosphorus removal from lagoon-pretreated swine wastewater by pilot-scale surface flow constructed wetlands planted with Myriophyllumaquaticum. Science of the Total Environment, 576:490-497.
Ma, J., Zhu, H., Fan, M. (2013). Distribution of heavy metals in pig farm biogas residues and the safety and feasibility assessment of biogas fertilizer. International Journal of Agricultural and Biological Engineering, 6(4):35-43.
Markou, G., Chatzipavlidis, I., Georgakakis, D. (2012). Cultivation of Arthrospira (Spirulina) platensis in olive-oil mill wastewater treated with sodium hypochlorite. Bioresource Technology, 112:234-241.
Mata, T., Martins, A., Caetano, N. (2010). Microalgae for biodiesel production and other applications: A review. Renewable and Sustainable Energy Reviews, 14(1):217-232.
Mazareli, R., Duda, R., Leite, V., et al. (2016). Anaerobic co-digestion of vegetable waste and swine wastewater in high-rate horizontal reactors with fixed bed. Waste Management, 52:112-121.
Meers, E., Tack, F., Tolpe, I., et al. (2008). Application of a full-scale constructed wetland for tertiary treatment of piggery manure: monitoring results. Water and Soil Poll, 193:15-24..
Miranda, AF., Ramkumar, N., Andriotis, C., et al. (2017). Applications of microalgal biofilms for wastewater treatment and bioenergy production. Biotechnology for Biofuels, 10.
Mitsch, WJ., Gosselink, JG. (2000). The value of wetlands: importance of scale and landscape setting. Ecological Engineering, 35 (1):25-33.
Molinuevo-Salces, B., Mahdy, A., Ballesteros, M., et al. (2016). From piggery wastewater nutrients to biogas: Microalgae biomass revalorization through anaerobic digestion. Renewable Energy, 96:1103-1110.
Møller, HB., Lund, I., Sommer, SG. (2000). Solid–liquid separation of livestock slurry: efficiency and cost. Bioresource Technology, 74(3):223-229.
Møller, HB., Sommer, SG., Ahring, BK. (2004). Methane productivity of manure, straw and solid fractions of manure. Biomass and Bioenergy, 26(5):485-495.
Nwoba, E.G., Ayre, J.M., Moheimani, N.R., et al. (2016). Growth comparison of microalgae in tubular photobioreactor and open pond for treating anaerobic digestion piggery effluent. Algal Res. 17:268-276
Peng, J., Song, Y., Liu, Z., et al. (2012). Performance of a novel Circular-Flow Corridor wetland toward the treatment of simulated high-strength swine wastewater. Ecological Engineering, 49:1-9.
Perazzoli, S., Bruchez, B., Michelon, W., et al. (2016). Optimizing biomethane production from anaerobic degradation of Scenedesmus spp. biomass harvested from algae-based swine digestate treatment. International Biodeterioration & Biodegradation, 109:23-28.
Poach, M., Hunt, P., Reddy, G., et al. (2004). Swine wastewater treatment by marsh–pond–marsh constructed wetlands under varying nitrogen loads. Ecological Engineering, 23(3):165-175.
Posten, C. (2009). Design principles of photo‐bioreactors for cultivation of microalgae. Engineering in Life Sciences, 9(3):165-177.
Posten, C., Schaub, G. (2009). Microalgae and terrestrial biomass as source for fuels—A process view. Journal of Biotechnology, 142(1):64-69.
Ra, C., Lau, A. (2010). Swine Wastewater Treatment Using Submerged Biofilm SBR Process: Enhancement of Performance by Internal Circulation through Sand Filter. Journal of Environmental Engineering-ASCE, 136(6):585-590.
Ramanan, R., Kim, BH., Cho, DH., et al. (2016). Algae-bacteria interactions: Evolution, ecology and emerging applications. Biotechnology Advances, 34(1):14-29.
Rawat, I., Kumar, RR., Mutanda, T., et al. (2011). Dual role of microalgae: Phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Applied Energy, 88(10):3411-3424.
Razzak, S., Hossain, M., Lucky, R., et al. (2013). Integrated CO2 capture, wastewater treatment and biofuel production by microalgae culturing—A review. Renewable and Sustainable Energy Reviews, 27:622-653.
Renuka, N., Sood, A., Ratha, SK., et al. (2013). Evaluation of microalgal consortia for treatment of primary treated sewage effluent and biomass production. Journal of Applied Phycology, 25(5):1529-1537.
Riaño, B., García-González, MC. (2014). On-farm treatment of swine manure based on solid–liquid separation and biological nitrification–denitrification of the liquid fraction. Journal of Environmental Management, 132:87-93.
Ruiz-Marin, A., Mendoza-Espinosa, L., Stephenson, T. (2010). Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresource Technology, 101(1):58-64.
Shin, PKS., Lam, NWY., Wu, RSS., et al. (2008). Spatio-temporal changes of marine macrobenthic community in sub-tropical waters upon recovery from eutrophication. I. Sediment quality and community structure. Marine Pollution Bulletin, 56(2):282-296.
Shin, S., Yoo, S., Hwang, K., et al. (2011). Dynamics of transitional acidogenic community along with methanogenic population during anaerobic digestion of swine wastewater. Process Biochemistry, 46(8):1607-1613.
Stone, K., Poach, M., Hunt, P., et al. (2004). Marsh-pond-marsh constructed wetland design analysis for swine lagoon wastewater treatment. Ecological Engineering, 23(2):127-133.
Sun, F., Sun, W.-L. (2012). A simultaneous removal of beryllium and ammonium–nitrogen from smelting wastewater in bench- and pilot-scale biological aerated filter. Chemical Engineering Journal, 210:263-270.
SundarRajan, P., Gopinath, K. P., Greetham, D., et al. (2019). A review on cleaner production of biofuel feedstock from integrated CO2 sequestration and wastewater treatment system. Journal of Cleaner Production, 210, 445-458.
Tao, W., Ukwuani, AT. (2015). Coupling thermal stripping and acid absorption for ammonia recovery from dairy manure: Ammonia volatilization kinetics and effects of temperature, pH and dissolved solids content. Chemical Engineering Journal, 280, 188-196.
Ugwu, C., Aoyagi, H., Uchiyama, H. (2008). Photobioreactors for mass cultivation of algae. Bioresource Technology, 99(10):4021-4028.
Vadivelu, VM., Keller, J., Yuan, Z. (2006). Effect of free ammonia and free nitrous acid concentration on the anabolic and catabolic processes of an enriched Nitrosomonas culture. Biotechnology and Bioengineering, 95(5):830-839.
Vadivelu, VM., Keller, J., Yuan, Z. (2006). Effect of free ammonia and free nitrous acid concentration on the anabolic and catabolic processes of an enriched Nitrosomonas culture. Biotechnol Bioeng, 95(5):830-839.
Wang, XJ., Xia, SQ., Chen, L., et al. (2006). Nutrients removal from municipal wastewater by chemical precipitation in a moving bed biofilm reactor. Process Biochemistry, 41(4):824-828.
Wang, Y., Guo, W., Yen, H., et al. (2015). Cultivation of Chlorella vulgaris JSC-6 with swine wastewater for simultaneous nutrient/COD removal and carbohydrate production. Bioresource Technology, 198:619-625.
Wang, Y., Ho, S., Cheng, CL., et al. (2017). Nutrients and COD removal of swine wastewater with an isolated microalgal strain Neochlorisaquatica CL-M1 accumulating high carbohydrate content used for biobutanol production. Bioresource Technology, 242:7-14.
Wang, Z., Dong, J., Liu, L., et al. (2013). Screening of phosphate-removing substrates for use in constructed wetlands treating swine wastewater. Ecological Engineering, 54:57-65.
Wang, Z., Liu, C., Liao, J., et al. (2014). Nitrogen removal and N2O emission in subsurface vertical flow constructed wetland treating swine wastewater: Effect of shunt ratio. Ecological Engineering, 73:446-453.
Wen, Y., He, Y., Ji, X., et al. (2017). Isolation of an indigenous Chlorella vulgaris from swine wastewater and characterization of its nutrient removal ability in undiluted sewage. Bioresource Technology, 243, 247-253. doi: https://doi.org/10.1016/j.biortech.2017.06.094
Wen, S., Liu, H., He, H., et al. (2016). Treatment of anaerobically digested swine wastewater by Rhodobacter blasticus and Rhodobacter capsulatus. Bioresource Technology, 222:33-38.
Xian, Q., Hu, L., Chen, H., et al. (2010). Removal of nutrients and veterinary antibiotics from swine wastewater by a constructed macrophyte floating bed system. Journal of Environmental Management, 91(12):2657-2661.
Xu, H., Miao, X., Wu, Q. (2006). High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. Journal of Biotechnology, 126(4):499-507.
Xu, J., Zhao, Y., Zhao, G., et al. (2015). Nutrient removal and biogas upgrading by integrating freshwater algae cultivation with piggery anaerobic digestate liquid treatment. Appl. Microbiol. Biotechnol. 99 (15):6493-6501.
Yang, D., Deng, L., Zheng, D., et al. (2015). Separation of swine wastewater into solid fraction, concentrated slurry and dilute liquid and its influence on biogas production. Fuel, 144:237-243.
Ye, J., Song, Z., Wang, L., Zhu, J. (2016). Metagenomic analysis of microbiota structure evolution in phytoremediation of a swine lagoon wastewater. Bioresource Technology, 219:439-444.
Zhu, L., Wang, Z., Shu, Q., et al. (2013). Nutrient removal and biodiesel production by integration of freshwater algae cultivation with piggery wastewater treatment. Water Research, 47(13):4294-4302.
Zhu, L., Wang, Z., Takala, J., et al. (2013). Scale-up potential of cultivating Chlorella zofingiensis in piggery wastewater for biodiesel production. Bioresource Technology, 137:318-325.