Oocystis borgei (Chlorophyta, Chlorophytina, Trebouxiophyceae, Chlorellales, Oocystaceae, Oocystis) is a unicellular algae which is widespread with stable population distribution, has a strong ability to adapt to the environment, and plays an important role in the regulation of the water environment for shrimp aquaculture. It can inhibit effectively the growth of Vibrio in the culture environment, absorb heavy metals and ammonia nitrogen (Huang et al., 2002a; Li et al., 2010; Huang et al., 2002b; Huang et al., 2012; Huang et al., 2002c; Liu et al., 2012). The industrial production of O. borgei has been completed in China. Previous studies have suggested that vitamins play an important role in the population structure of algae (Swiftdg, 1984). They have the effect of promoting the growth and reproduction of red tide organisms. Different algae have different correlations for Vitamins B1 and B12 (Xie, 2000). Vitamin B1 is involved in the metabolism of algal cells in vivo. It is an important coenzyme in the aerobic metabolism of carbohydrates, which helps the algae cells to release free energy for cell division and proliferation (Lei et al., 2010). Vitamin B12 has a certain role in promoting the growth of some algae (Zhu et al., 1994). In addition, Vitamins (B1, B12) could significantly promote the proliferation of Heterosigma akashiwo, Haematococcus plvialis, and Dunaliella salina (Hu et al., 2003; Zhang et al., 2004; Li et al., 2009; Guo et al., 2012). Previous reports on microalgae polysaccharides show that cell polysaccharides are the material basis of the microalgal formation population (Yang et al., 2006). Under as tressed condition, Microcystis can secrete a large amount of extracellular polysaccharides, by which single algal cells are grouped to defense against the grazer (Yang et al., 2006). In the same way, Achnanthesbrevipes, Chaetocerosaffinis, Porphyridium sp, and Phaeodactylum tricornutum may also produce a large number of intracellular and extracellular polysaccharides, allowing the algae cells to survive an adverseenvironment (Abdullahi et al., 2006; Myklestad et al., 1972; Gyerrini et al., 2000; Arad et al., 1988).
The current research on O. borgei focuses on its growth, ecological effects, and applications. Some researchers studied the growth and metabolism of O. borgei of the absorption of heavy metals of Cu2+ and Zn2+at different concentrations (Li et al., 2010; Huang et al., 2002b; Huang et al., 2012; Huang et al., 2002c). Others studied the absorption rate of ammonia nitrogen of O. borgei under different conditions (Liu et al., 2012). However, no report is available on the requirement of Vitamin B1 and Vitamin B12 for the growth of O. borgei.
This experiment used different concentrations of Vitamin B1 and Vitamin B12to culture O. borgei. The optimum growth conditions were determined in terms of growth rate and photosynthetic pigment content. At the same time, by measuring the cell polysaccharide content in different growth conditions, we explored the relationship between the production of polysaccharides and the growth status, which provides a theoretical basis for the directional cultivation of O. borgei.
2. Materials and Methods
2.1 Experiment algae and tissues
The algae used in this study were obtained from the Key Laboratory of Aquaculture in South China Sea for Aquatic Economic Animal of Guangdong Higher Education Institutes, and the Laboratory of Ecology of Water Area and Aquaculture Environment of WHAT ORGANIZATION?, WHAT COUNTRY?. The seawater used was obtained from the sea area of Dongfeng Wharf in Zhanjiang, Guangdong, sand-filtered and filtered through a 400-mesh sieve (mixed fiber membrane), then sterilized by autoclaving.
The same amount of algae that is in the growth index period was centrifuged at 3000 r/min for 10 min to remove the supernatant and inoculated into the culture solution. The composition of the culture solution was formulated with Zhanshui 107-13 culture solution (Chen, 1995). The culture bottle is 500 mL conical flask, added 400 mL of culture solution. The O. borgei were maintained at 25 ± 1 °C in a humidified incubator under 2000 lx light intensity by fluorescent lamps in light and dark cycle of12 h׃12 h continuously for 15 days, from 7:00 to 23:00, shaking every four hours.
2.2 Nutrient concentration setting
Vitamin B1 was divided into five triplicated groups in graded concentrations of 0.1, 0.5, 1, 2, 4 mg/L, respectively. The control group contained zero Vitamin B1. So did for Vitamin B12 except for the unit in μg/L.
2.3 Cell density counting method
The relationship between algal cell density and optical density values was investigated by diluting the algal fluid used in the experiment into different concentration gradients. The absorbance (OD) at a wavelength of 650 nm was measured with a Shimadzu UV-2450 spectrophotometer and expressed as OD650. The diluted algal fluid was counted in the hemocytometer and processed by the Microsoft Office Excel. The results show a good linear relationship between OD650 and algal cell density (C) in the regression equation of C = 748.34OD650 + 6.3245.
From the second day onward, sampling was performed at regular intervals of every two days. The Shimadzu UV-2450 spectrophotometer was used to measure the OD650. The same culture fluid as the non-algae species was used as a blank control. Calculation method of relative growth constant K = (lgODt– lg OD0 )/Tin which OD0is the absorption of algae cells at the start of culture, ODt is that of the culture end, and Tforculture time in day (Li, 2007). This experiment calculated the relative growth constant on the last day.
2.4 Measurement methods of chlorophyll a, chlorophyll b, carotenoids
Concentrations of chlorophylls a and b, and carotenoid in algae measured were calibrated as peril (2007). The last day of the experiment was carried out by taking 3 mL of algal fluid and centrifuged at 3000 r/min for 10 min, the supernatant was discard, then centrifuged under the same conditions for 5 min. Add 95% alcohol to 5 mL, shaken, then kept in dark and 4 ℃. Shaken every 12 h until the algae became white (need about 24 h to 48 h), then centrifuge at 4000 r/min. Measure the absorbance at 665, 649, 470nm with the Shimadzu uv-2450 spectrophotometer.
Chlorophyll a content (μg/L): Chla = 13.95A665– 6.88A649,
Chlorophyll b content (μg/L): Chlb = 24.96A649– 7.32A665,
Carotenoid content (μg/L): Car= (1000A470– 2.05Chla– 114.8Chlb)/245 (Li, 2007).
2.5 Extraction of polysaccharides from algal cells
In this experiment, the extraction of polysaccharides by hot water extraction was slightly improved from that of Mu (2010). Since most of the polysaccharides have a high solubility in hot water, this method can be used to obtain stable and high yield in minimal damage when extracting polysaccharides (Zou, 2015). On the last day of culture, 3-mL of algal fluid was centrifuged at 3000 r/min for 8 min. The supernatant was removed and centrifuged under the same conditions for 5 min. Two supernatants were taken as extracellular soluble polysaccharides.
Afterwards, algae cells were dried as much as possible, 3-mL 0.9% NaCl solution was added, and heated in80 °C water bath for 6 h. The mixture was shaken hourly to obtain the extract. After cooling, supernatant was centrifuged at 4000 r/min and added with3% TCA solution to detect whether a precipitate of protein remained. The supernatant obtained after removing the precipitated protein was the extracellular algae-fixed polysaccharide solution.
2.6 Preparation of cellulose calculation standard curve
Standard glucose solutions 0.0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, and 1.0 mL were accurately pipetted in test tubes and added with water to a total volume of 2.0 mL per tube; and 2.0-mL water was set as the blank control. Phenol-sulfuric acid method (Bai et al., 2004) was adapted. Briefly, 1.0-mL 0.6% phenol solution was added into each test tube, mixed, added with 5.0-mL concentrated sulfuric acid, and heated in a boiling water bath for 10 min. After cooled, the absorbance at 490 nm was measured and standard curves drawn.
Since the algae use seawater, the extracellular soluble polysaccharides are secreted polysaccharides dissolved in seawater, and the extracellular polysaccharides are extracted using freshwater extraction. Therefore, the standard curve of seawater (consistent with seawater used for algae cultivation) and fresh water (distilled water) was done once. The content of the extracellular soluble polysaccharide ysp can be calculated by: ysp = 0.1757A490 + 0.00238, and that of the extracellular fixed polysaccharide yfp by: yfp = 0.19087A490–0.00031. The total amount of polysaccharides thus were measured and divided by the number of cells to obtain extracellular soluble polysaccharides and extracellular immobilized polysaccharides contained in each cell.
2.7 Statistical analysis
Data were analyzed by One-Way ANOVA followed by the Tukey multiple comparison test using the SPSS23.0 software. Significance was set at P< 0.05.
3. Results
3.1 Effect of Vitamin B1 on the Growth of O. borgei
From the growth curve (Fig.1a), it can be seen that in the first 2 days, the amount of cells in each group was not significantly different. At the beginning, the biomass of alga cells in the group of Vitamin B1 concentration at 0.1 to 2 mg/L was gradually higher than that of the control group and then entered the exponential growth phase. Therefore, the concentration of Vitamin B1 at 0.5 mg/L was the optimum concentration for the culture of O. borgei. When the concentration of Vitamin B1 was above 4 mg/L, the growth of O. borgei was inhibited.
From the relative growth constant K, the effects of different concentrations of Vitamin B1 on the relative growth constant of O. borgei are extremely significant (Fig.1b). With the increase of Vitamin B1 concentration, the value of K increased first and then decreased. In the Vitamin B1concentration of 0.5 mg/L, the value of K reached the maximum increase by36.8% from the control group, which is the optimal culture concentration in which O. borgei grew the fastest. In the Vitamin B1concentration of above 4 mg/L, K value decreased, and the growth of O. borgei was inhibited. Multiple comparison analysis showed significant differences among groups.
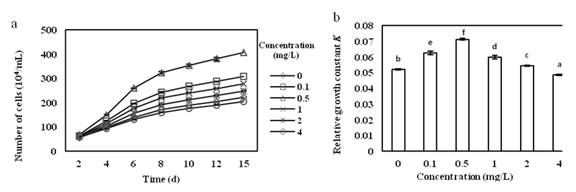
Fig.1 (a) Effects of different concentration of Vitamin B1 on the growth of O. borge. (b) Effects of different concentration of Vitamin B1 on relative growth constant of O. borge
3.2 Effect of Vitamin B1 on Cytochrome Content of O. borgei
The effects of different concentrations of Vitamin B1 on the synthesis of chlorophyll a in O. borgei were extremely significant (Fig.2a). With the increase of Vitamin B1 concentration, the photosynthetic pigment content of O. borgei increased first and then decreased. In Vitamin B1 concentration of 0.5 mg/L, the content of chlorophyll a reached the maximum of 0.845 μg/L. In the concentration of Vitamin B1above 4 mg/L, the chlorophyll a synthesis of O. borgei was inhibited to a minimum value. Multiple comparison analysis showed that in Vitamin B1concentration of 0.5 mg/L, the chlorophyll a content varied the most among groups.
The effect of different concentrations of Vitamin B1 on the synthesis of chlorophyll b in O. borgei was extremely significant (Fig.2b). In Vitamin B1concentration of 0.5 mg/L, the content of chlorophyll b reached them aximum of 0.297 μg/L. The multiple comparison analysis showed that in Vitamin B1concentration of 0.5 mg/L, the chlorophyll b content was the most different among groups.
The effects of different concentrations of Vitamin B1 on the synthesis of carotenoids in O. borgei were extremely significant (Fig.2c). In Vitamin B1concentration of 0.5 mg/L, the content of carotenoid reached the maximum of 0.235 μg/L. Multiple comparison analysis showed that the difference in carotenoid content of Vitamin B1 at 0.5 mg/L was the most significant among groups.
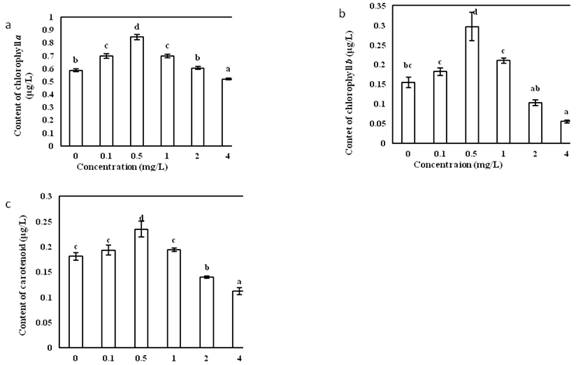
Fig.2 (a) Effects of different concentration of Vitamin B1 on chlorophyll a inO. borge. (b) Effects of different concentration of Vitamin B1 on chlorophyll b inO. borge. (c) Effects of different concentration of Vitamin B1 on carotenoid content inO. borge
3.3 Effect of Vitamin B1 on the polysaccharide contents of O. borgei
The effect of different concentrations of Vitamin B1 on the content of extracellular polysaccharides in the single cells of O. borgei was extremely significant (Fig.3a). As the concentration of Vitamin B1 increased, the content of extracellular fixed polysaccharides in the single cells of O. borgei was decreasing first and then rising. When the concentration of Vitamin B1 was 0.5 mg/L, it reached the lowest at 0.0019 pg/cell. When the concentration of Vitamin B1 was 4 mg/L, the content of extracellular fixed polysaccharide was the highest, reaching 0.0061 pg/cell, which is 3.2 times of the minimum value. Multiple comparison analysis showed that in Vitamin B1concentration of 0.5 mg/L, the content of extracellular fixed polysaccharides was most significantly different among groups.
The effect of different concentrations of Vitamin B1 on the content of extracellular soluble polysaccharides in single cells of O. borgei was extremely significant (Fig.3b). As the concentration of Vitamin B1 increased, the content of extracellular soluble polysaccharides in single cells of O. borgei first decreased and then increased. The content of extracellular soluble polysaccharide was the lowest in Vitamin B1 concentration of 0.5 mg/L, being 0.0014 pg/cell, and the content of extracellular soluble polysaccharide was the highest at Vitamin B1 concentration of 4 mg/L, reaching 0.0022 pg/cell that is 1.57 times of the minimum. Multiple comparison analysis showed that the content of extracellular soluble polysaccharide in Vitamin B1 at 0.5 mg/L showed the most significant difference among all groups.
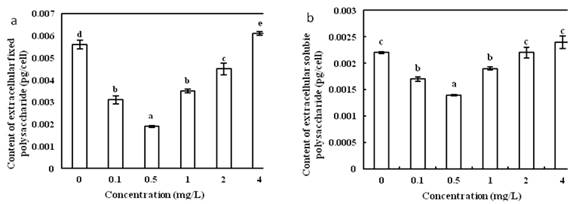
Fig.3 (a) Effects of different concentration of Vitamin B1 on the extracellular fixed polysaccharides content of O. borge. (b) Effects of different concentration of Vitamin B1 on extracellular soluble polysaccharide content of O. borge
3.4 Effects of Vitamin B12 on the growth of O. borge
The growth curve (Fig.4a) of the first 2 days was not significantly different among groups. At the beginning, the biomass of alga cells in the group of Vitamin B12 concentrations of 0.1 to 4 μg/L was gradually higher than that of the control group and entered the exponential growth phase. The concentration of Vitamin B12 at 2 μg/L was the optimum concentration to culture O. borgei.
The relative growth constant K was extremely and significantly different in Vitamin B12 concentration groups (Fig.4b). With the increase of Vitamin B12 concentration, the K value showed a trend of uprising first and then down. In Vitamin B12concentration of 2 μg/L, the K value reached the maximum, which an increase of 36.2% was compared with the control group, so this is its optimal culture concentration and O. borgei grew the fastest. Multiple comparison analysis showed that in Vitamin B12 concentration of 2 μg/L, the among-group difference was the greatest.
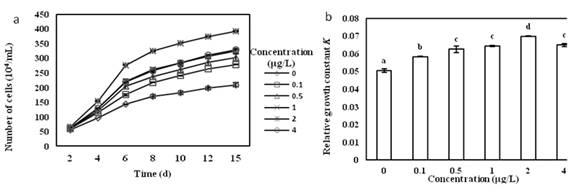
Fig.4 (a) Effects of Different Concentration of Vitamin B12 the Growth of O. borge. (b) Effects of Different Concentration of Vitamin B12relative growth constantof O. borge
3.5 Effect of Vitamin B12 on cytochrome content of O. borgei
The effects of different concentrations of Vitamin B12 on the synthesis of chlorophyll a in O. borgei was extremely significant (Fig.5a). With the increase of Vitamin B12 concentration, the chlorophyll a content increased first and then decreased. When the concentration of Vitamin B12 was 2 μg/L, the content of chlorophyll a reached a maximum of 0.726 μg/L, showing the strongest promotion. Multiple comparison analysis showed that in Vitamin B12 concentration of 2 μg/L the among-group difference was most significant.
The effects of different concentrations of Vitamin B12 on the synthesis of chlorophyll b in O. borgei were extremely significant (P < 0.01). In Vitamin B12 concentration of 2 μg/L, chlorophyll b reached the maximum of 0.3096 μg/L, which had the strongest promoting effect. Multiple comparison analysis showed that in Vitamin B12 concentration of 2 μg/L the among-group difference was most significant.
The effects of different concentrations of Vitamin B12 on the synthesis of carotenoids in O. borgei were significant (Fig.5b). In Vitamin B12concentration of 2 μg/L, carotenoid content reached the maximum of 0.1823 μg/L, showing the strongest promotion. Multiple comparison analysis showed that in Vitamin B12 concentration of 2 μg/L the among-group difference was most significant.
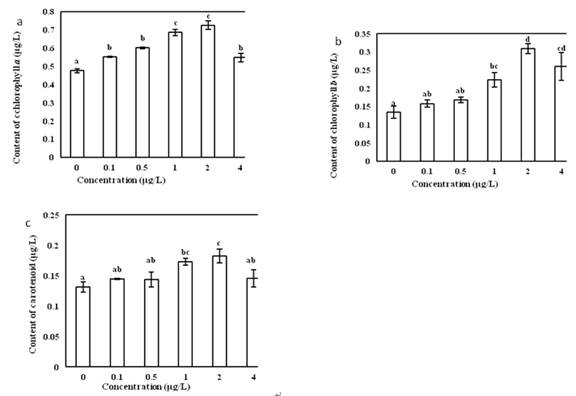
Fig 5 (a) Effects of different concentration of Vitamin B12 on chlorophyll a in O. borge. (b) Effects of different concentration of Vitamin B12 on chlorophyll b in O. borge. (c) Effects of different concentration of Vitamin B12 on carotenoid content in O. borge
3.6 Effect of Vitamin B12 on the content of polysaccharides of O. borgei
The effect of different concentrations of Vitamin B12 on the content of extracellular fixed polysaccharides in the single cells of O. borgei was extremely significant (Fig.3a). With the increased of the concentration of Vitamin B12, the content of extracellular fixed polysaccharides in the single cells of O. borgei dropped first and then rose. In Vitamin B12 concentration of 2 μg/L, extracellular fixed polysaccharides was the lowest at 0.002 pg/cell. With no Vitamin B12 adding, the content of extracellular fixed polysaccharides was the highest, reaching 0.0052 pg/cell, which is 2.6 times of the minimum. Multiple comparison analysis showed most significant among-groups differences in Vitamin B12 concentration of2 μg/L.
The effect of different concentrations of Vitamin B12 on the content of extracellular soluble polysaccharides in single cells of O. borgei was extremely significant (Fig.3b). As the concentration of Vitamin B12increased, the content of extracellular soluble polysaccharides in single cells of O. borgei decreased first and then increased. The content of extracellular soluble polysaccharides was the lowest in Vitamin B12concentration of 2 μg/L down to 0.0012 pg/cell. Without Vitamin B12adding, the content of extracellular soluble polysaccharides was the highest to 0.0024 pg/cell. It is 2 times of the minimum value. Multiple comparison analysis showed that in the Vitamin B12 concentration of 2 μg/L, the among-group difference was the most significant.
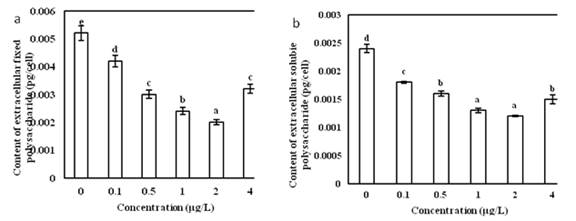
Fig.6 (a) Effects of different concentration of Vitamin B12 on the extracellular fixed polysaccharides content of O. borge. (b) Effects of different concentration of Vitamin B12 on the extracellular soluble polysaccharides content of O. borge
4. Discussion
4.1 Effects of Vitamin B1 on the growth of O. borgei
In eukaryotic cells, Vitamin B1 is thiamine monophosphate, which is formed by the polymerization of two phosphates (Lei et al., 2010). It is converted into thiamine pyrophosphate (also known as a secondary carboxylase) in the body and is involved in the metabolism of algal cells. The synthesis of photosynthetic pigments increased and promoted the cell growth and increase (Guo et al., 2012). In addition, Vitamin B1 is an important coenzyme in the aerobic metabolism of carbohydrates. The aerobic decomposition of carbohydrate is much greater than the free energy released when anaerobic decomposition occurs. Therefore, the aerobic decomposition of carbohydrates provides a large amount of free energy to algae to split proliferation and growth more rapidly (Lei et al., 2010). Studies have shown that Vitamin B1 has a significant effect on the proliferation of populations of GY-H36 Karenia mikimotoi. When the concentration exceeds 3.125 μg/L, Vitamin B1 can significantly promote the proliferation of GY-H36 Karenia mikimotoi and the maximum population density is significantly increased (Lei et al., 2010). The appropriate amount of Vitamin B1 can promote the growth of Haematococcus pluvialis and the production of astaxanthin (Zhang et al., 2004). Vitamin B1 can increase the photosynthetic pigment formation of Dunaliella Salina, increase the accumulation of protein, and accelerate cell reproduction (Guo et al., 2012). Vitamin B1 can promote the growth of D. salina (Hu et al., 2003). Toshitaka et al. (1978) and Wang et al., (1996) believe that Vitamin B1 has a significant effect on the growth and reproduction of certain microalgae species. The results of this experiment show that the concentration of Vitamin B1 in the range from 0.1 to 2 mg/L had the positive effect on the growth of O. borgei. With the increase of the concentration, the relative growth of O. borgei, chlorophyll a, chlorophyll b, and carotenoid content all increased first. The decreasing trend showed that when the concentration was greater than 0.5 mg/L, the relative growth of O. borgei was the highest, which is the optimum concentration of culture. When the concentration was greater than 4 mg/L, biomass of the algae was no better than that without vitamins. The algal biomass of the Vitamin B1 control group was large. It can be inferred that low concentration of Vitamin B1 is conducive to promoting the growth and metabolism of O. borgei and can increase its photosynthetic pigment production, while an excessively high concentration of Vitamin B1would inhibit the synthesis of pigment proteins in O. borgei. Therefore, it is necessary to add Vitamin B1 to the O. borgei directional cultivation. The concentration of Vitamin B1 is controlled within the range of 0.1 to 2 mg/L, which can promote the O. borgei to a certain extent.
4.2 Effect of Vitamin B12 on the growth of O. borgei
Vitamin B12 contains cobalt, also known as cobalamin, and its structure is very complex. In addition to the cobalt atom, the molecule also contains 5,6-dimethylbenzimidazole, 3'-phosphate ribose, aminopropanol, and porphyrin ring components (Nie et al., 1999). Porphyrin compounds form the core part of chlorophyll and other biological macromolecules and participate in a series of important biochemical processes such as photosynthesis of plants, which can enhance the photosynthesis of plants by promoting the production of chlorophyll in the plant kingdom. Studies have shown that Vitamin B12 is needed for the growth of microalgae (Swiftdg, 1984; Yamochis, 1984), and Vitamin B12 can promote the proliferation of Toxic dinoflagellate with a half-saturation rating of 0.22 ng/L and a maximum specific growth rate of 0.55/d (You, 2002).In the concentration of complex Vitamin B12at 1.0 μg/L, the maximum growth rate of GY-H31 Alexandrium tamarense was 0.26/d (Jiang et al., 2006), and Vitamin B12 had a certain role in promoting the growth of other algae (Zhu et al., 1994). At the same time, there are also literatures showing that the acquisition of Vitamin B12 by algae can be obtained through symbiosis with bacteria (Croft et al., 2005), and that Vitamins are not necessary for the proliferation of certain algae (Pang et al., 2006). For example, Vitamin B12 has no significant effect on the proliferation of Chlorella (Hu et al., 2003). The types of microalgae used in these experiments are different, and the optimal concentration of Vitamins is different even if they affect the proliferation of microalgae (Wang et al., 1996; Li et al., 2000; Wen et al., 2002). This also reminds us that we need to pay attention to the selection of algae cell types when exploring the use of Vitamins as a nutrient additive. The results of this experiment show that the concentration of Vitamin B12 was set in the range of 0.1–2 μg/L. With the increase of the concentration, the relative growth of O. borgei, chlorophyll a, chlorophyll b, and carotenoid contents of O. borgei tended to reduce. It can be inferred that the addition of an appropriate amount of Vitamin B12 is beneficial to promote the growth and metabolism and the production of photosynthetic pigments of O. borgei. Therefore, it is necessary to add Vitamin B12 to the growth of O. borgei in order to control the growth of it. Concentration is controlled within the range of 0.1 ~ 2 μg/L, which can promote the O. borgei to a certain extent, and growth and synthesis of pigment proteins.
4.3 Effect of Vitamin B1 on cellular polysaccharide synthesis in O. borgei
Vitamin B1 is a very important coenzyme in the aerobic metabolism of carbohydrates (Lei et al., 2010). Vitamin B1 can promote the aerobic decomposition of polysaccharides and reduce the accumulation of polysaccharides in the algae cells. Aerobic decomposition of carbohydrates generates a large amount of free energy to promote the growth and reproduction of algae cells, and the strong division of algae cells results in a decrease in the accumulation of polysaccharides in individual cells. At present, the effect of Vitamin B1 on the polysaccharide content of microalgae has not been reported. However, in this study, with the increase of the Vitamin B1 concentration in the range of 0.1 to 0.5 mg/L, the concentration of extracellular fixed polysaccharides and extracellular soluble polysaccharides in the single cells of O. borgei increased. The contents of these two polysaccharides showed a tendency of decreasing first and then increasing. The content of the two polysaccharides was the lowest when the concentration of Vitamin B1 was 0.5 mg/L, and the content of the two kinds of polysaccharides reached the highest when the concentration of Vitamin B1 was 4 mg/L and the control group that contained no Vitamin B1. Therefore, it can be inferred that when O. borgei is cultured in a medium supplemented with Vitamin B1, a high concentration of Vitamin B1 promotes the accumulation of polysaccharides in the cells of O. borgei, thereby protecting the algae and allowing algae to survive in the bad environment. It follows that the polysaccharide production of O. borgei is inconsistent with the requirement for the growth of Vitamin B1.
4.4Effect of Vitamin B12 on cellular polysaccharide synthesis in O. borgei
Vitamin B12 is a porphyrin compound containing metal cobalt (Nie et al., 1999) and is an important substance involved in physiological and biochemical reactions in organisms. Porphyrin compounds form the core of chlorophyll and other biological macromolecules, thus can enhance the photosynthesis of plants by promoting the production of chlorophyll in the plant kingdom. The product of photosynthesis of Vitamin B12 is preferentially expressed in the form of protein and RNA, while the content of polysaccharide is relatively reduced. In conditions conducive to the proliferation of algal cells, algae cells preferentially use energy to produce RNA and proteins, and the amount of energy storage material that can be produced is reduced (Philippis et al., 1993; Yang et al., 2008). At present, the effect of Vitamin B12 on the polysaccharide content of microalgae has not been reported. However, we find that in the concentration of Vitamin B12 in the range of 0.1–2 μg/L, the extracellular fixed polysaccharides and extracellular soluble polysaccharides in the single cells of O. borgei were downregulated. The contents of the two polysaccharides showed a tendency of decreasing first and then increasing, and reached the lowest values in Vitamin B12concentration 2 μg/L. In the control group in which Vitamin B12 was not added, the content of both polysaccharides was the highest. Therefore, it can be inferred that adding a high or low concentration of Vitamin B12 in the culture of O. borgei can promotes the accumulation of polysaccharides in the cells of it. O. borgei is able to adapt to an adverse ambiance by increasing the secretion of polysaccharides. Studying on the demand of Vitamin B12 showed that the polysaccharide production by O. borgei was contrary to its growth.
4.5Relationship between polysaccharide production and stress resistance of O. borgei
Currently, study of microalgae polysaccharides focused on animal and human-related activities, and there are few reports on the activity of plants and their anti-adversity effects. The secretion of extracellular polysaccharides from microalgae is closely related to nutrient levels and growth status (Dai et al., 2013). When will the amount of extracellular polysaccharide release to the maximum? The answer varies among the scientists. One opinion is that the production and secretion of polysaccharides from Chaetocerossp and Thalassiosira pseudonana are mainly in quiescent phase, while Phaeodactylum tricornutum and Nitzschia closterium can produce and excrete higher polysaccharides in the early and stationary phase of exponential growth (Wang et al., 2003).
Under conditions of high salinity and limited N and P nutrient, the yield of extracellular soluble polysaccharides increased significantly in Amphoracoffeaeformis. The higher the salinity, the higher the yield of extracellular polysaccharides and the higher the yield under the stressed conditions. The polysaccharide content is 2 times that of the polysaccharide produced under the optimal conditions (the yield increased from 0.67 to 1.34 pg/cell), and the yield of the extracellular polysaccharides of benthic diatom Amphoracoffeaeformis increased under the limitation of N and P nutritions (Ma et al., 2008). In the process of cultivation of saltern benthic diatom Navicula lanceolata, the content of extracellular soluble polysaccharides produced under the stress conditions was 1.13 times that produced under optimal conditions (the yield of extracellular polysaccharides was increased from 5.09 to 5.74 pg/cell) (Ma et al., 2009). This experiment showed that under the conditions of suitable Vitamin B1 and Vitamin B12 concentrations, both extracellular soluble polysaccharides and extracellular fixed polysaccharides produced by the algal cells had a significant decreasing tendency. In the absence or high concentration of Vitamin B1, the content of extracellular polysaccharides produced by individual cells increased from 0.0018 to 0.0061 pg/cell as the stressed conditions intensified and the polysaccharide content produced was 3.2 times that of the optimal condition. In the absence of Vitamin B12 culture conditions, as the stressed conditions intensified, the content of extracellular polysaccharides produced by single cells increased from 0.002 to 0.0052 pg/cell, and the polysaccharide content produced under stress conditions was 2.6 times that of the optimal condition. Therefore, O. borgei accumulates polysaccharides more easily than other algae in the stressed conditions.
Microcystiscan form a defensive aggregate of dozens to hundreds of cells from single cell to defend the grazer(Yang et al., 2006), by which viscosity of its surface would increase by increasing extracellular polysaccharides segregation (Yang, 2006). Phormidiumautumnale also has the similar system that extracellular polysaccharide secretions are significantly increased under intense grazing pressure by protozoa (Pajdak et al.,2001). In addition, adding appropriate amounts of N, P nutrients in the culture fluid of Microcystis can increase its growth rate but reduce its total polysaccharides production while allowing it to appear as a single cell rather than as a population (Dai et al., 2013). The extracellular polysaccharides secreted by the algal cells are beneficial to the formation of algal cell populations, and at the same time enhance its ability of self-protection. In adverse environments, algae cells secrete polysaccharides outside to protect themselves, allowing the algae cells to produce more polysaccharides during the dormant period. In the absence of Vitamins B1 and B12, the O. borgei can produce a large amount of polysaccharides, which proves that O. borgei have strong resistance, and they have sustained and stable biological characteristics in the high-density shrimp culture environment.
References
Abdullahi, A.S., Underwood, G.J.C, Gretz, M.R. (2006). Extracellular matrix assembly in diatoms (Bacillariophyceae). V. Environmental effects on polysaccharides synthesis in the model diatom Phaeodactylum tricornutum. Journal of Pharmacology. 42:363-378.
Arad, S., Friedman, O., Rotem, A. (1988). Effect to nitrogen on polysaccharide production in a Porphyridium sp, Applied and Environmental Microbiology. 54:2411-2414.
Bai, X.J., Su, J.Y., Zhao S.X., et al. (2004). Comparative study on the determination method of polysaccharide content in the cell culture medium of Nostocflagelliforme. Food Industry Data Science. 11(045):146.
Chen, M.Y. (1995). Biological Feed Culture: Agricultural Press, Beijing. 69-75.
Croft, M.T., Lawrence, A.D., Rauxdeery, E., et al. (2005). Algae acquire Vitamin B12 through a symbiotic relationship with bacteria. Comparative Biochemistry & Physiology Part A Molecular & Integrative Physiology. 146 (4):222.
Dai, X.X., Zhu, W., Li, M. (2013). Effects of nutrients on components and polysaccharide content of Microcystis cell. Journal of Lake Science. (02):277-282.
Guo, J.Y., Yang, X.L., Qin, J., et al. (2012). Effects of VB1 on cells growth of Dunaliella salina. Journal of Anhui Agricultural Sciences. (36):17593-17594.
Gyerrini, F., Cangini, M., Boni, L. (2000). Metabolic responses of the diatom Achnanthes brevipes (Bacillariophyceae) to nutrient limitation. Journal of Pharmacology. 36:882-890.
Hu, G.K., Zhang, Q.T., Dong, S.L. (2003). Study on effects of 3 kinds of vitamin on Heterosigma akashiwo growth. Journal of Tianjin Institute of Light Industry. (04):14-17.
Huang, X.h,, Wang, Q.h., (2002). A study on dominant phytoplankton species in high-level prawn ponds and its formation cause. Journal of Tropical Oceanography. (04):36-44.
Huang, X.H., Li, C.L., Liu, H.J., et al. (2002). Effects of plant growth regulators on the growth of Oocystis borgei. Transactions of Oceanology and Limnology. (03):46-52.
Huang, X.H., Li, C.L., Liu, C.W., et al. (2002). Studies on the Ecological Factors of Oocystis borgei. Journal of Zhanjiang Ocean University. (03):8-12.
Huang, X.H., Wei, S.H., Zhou, M.H., et al. (2012). Adsorption and tolerance of Cu2 +and Zn2 +by Oocystis borgei. Journal of Shanghai Ocean University. (03):374-381.
Lei, Q.Y., Lu, S.H. (2010). Effects of Vitamin B1 and B12 on Reproduction of Karenia kimotoi. Journal of Anhui Agricultural Sciences. (18):9753-9755 + 9791.
Li, H.F., Zhou, H.Q., (2000). Effects of nutrients on growth, and content of total lipid and EPA of chlorella SP-2. Studia Marine Sinica, (00):55-64.
Li, H.S. (2007). Principles and Techniques of Plant Physiology and Biochemistry Test, Higher Education Press, Beijing.
Li, J.H., Huang, X.H., Li, S.D. (2010). Effects of Oocystis borgei on growth of Vibrios. Journal of Guangdong Ocean University. (03):33-38.
Li, L.X., Duan Q.S., Zhan, Y. (2009). Effect of combined additions of vitamin on growth and astaxanthin accumulation of Haematococcus pluvialis. Journal of Heilongjiang Institute of Science and Technology. (01):12-15.
Liu, M., Huang X.H., Li, C.L., et al. (2012). Uptake rate of ammonium by Oocystis borgei under different conditions. Journal of Guangdong Ocean University. (01):29-34.
Ma, M.R., Li, P.F., Chen, L., et al. (2008). Effects of salinity and nutrient limitation on the growth and exopolysaccharide production of the benthic diatom Amphora coffeaeformis in Saltpan. Salt Industry & Chemical Industry. 37(05):30-34.
Ma, M.R., Li, P.F., Chen, L., et al. (2009). Effects of salinity and nutrient limitation on growth and extracellular polysaccharide of the saltern benthic diatom Navicula lanceolata. Transactions of Oceanology and Limnology. 1:95-102.
Mu, W.J. (2010). Studies on extraction process of polysaccharides of Spirulina (Arthrospira) platensis and its oxidation resistance to Drosophila melanogaster, Inner Mongolia Normal University, China.
Myklestad, S., Haug, A. (1972). Production of carbohydrates by the Marine diatom Chaetoceros affinis var. willei (Gran) Hustedt I. Effect of the concentration of nutrients in the culture medium, Journal of Experimental Marine Biology Ecology. 9:125-136.
Nie, J.C., Wu, G.L., Zhang, Y.S. (1999). Concise Textbook of Biochemistry, Higher Education Press, Beijing. 123-132.
Pang, B.Y., Wang, X.K., Sun, Z.N., et al. (2006). Effects of different factors on growth of Gymnodinium spp. Biotechnology. 16(1):63-65.
Pajdak, S.A., Fialkowska, E., Fyda, J. (2001). Phormidium autumnale (Cyanobacteria) defense against three ciliate grazer species. Aquatic Microbial Ecology. 23:237.
Philippis, R.D., Margheri, M.C., Pelosi,E., et al. (1993). Exopolysaccharide production by a unicellular cyanobacterium isolated from a hypersaline habitat. Journal of Applied Phycology. 5:387-394.
Swiftdg. (1984). Algal Assays for Vitamins, Shubertleal Gae as Ecological Indicators. Academic Press. 281-313.
Toshitaka, N., Ryoji, S., Yoshihiko, H. (1978). Production of Vitamin B12, Thiamine an Biotin by Freshwater Phytoplankton, Bulletin of the Japanese Society of Scientific Fisheries. 45(2):199-204.
Wang, D.Z., Huang, S.Y., Yan, W.G. (2003). Influences of nutrient status on extracellular carbohydrate production of marine planktonic diatoms. Journal of Oceanography in Taiwan Strait. 22(4):487-492.
Wang, Z.F., Zhang, Q., Lu, Y., et al. (1996). The effects of nutrients, vitamins and trace metals on the growth of the red tide organism Prorocentrum micans, Donghai Marine Science. 14(3):33-38.
Wen, Z.Y., Jiang, Y., Chen, F. (2002). High cell density culture of the diatom Nitzschia laevis for eicosapentaenoic acid production: fed-batch development, Process Biochemistry. 37:1477-1453.
Xie, J.M. (2000). Red Tide, Vitamin B1 and B12 in Dapeng Bay. Chinese Journal of Ecology. 19(4):46-49.
Yamochis. (1984). Nutrient factors involved in controlling the growth of red tide flagellates: Procentrum micans, Eutresphella sp, and Ghattonella marina in Osaka Bay. Bulletin of Plankton of Japanese Society. 31(2):97-106.
Yang, Z., Kong, F.X., Shi, X.L. (2006). Morphological response of Microcystis aeruginosa to grazing by different sorts of zooplankton. Hydrobiologia. 56:225-230.
Yang, Z., Li, J.J. (2008). Effects of abiotic factors on algae extracellular polysaccharide content. Chinese Journal of Applied Ecology. 19(1):198-202.
Yang, Z. (2006). Response of Algae to Grazing Pressure by Zooplankton. PhD Thesis. Nanjing: Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences, China.
You, Z.C. (2002). Effects of B Vitamins on Toxic Vortex Flagella. Fisheries Science & Technology Information. 29(6):72.
Jiang, T.J., Xu, Y.X. (2006). Study on the growth of Alexandrium tamarense Balech, Acta Hydrobiologica Sinica. 30(4):472-476.
Zhang, Y., Cai, M.G., Qi, A.X., et al. (2004). The effect of Vitamin B1, B12 on the two culture stage of Haematococcus plvialis. Journal of Xiamen University (Natural Science). (S1):142-146.
Zhu, C.G., Qi, Y.Z., Guo,C.B. (1994). The effect of iron, Vitamins B1, B12, nitrogen and phosphorus on the growth of Prorocentrum micans. Oceanologia et Limnologia Sinica. 25(2):168-172.
Zou, S. (2015). Extraction and separation of polysaccharides from bergamot and their antioxidant activities in Vitro. Chongqing University, China.