
Fig.1 The investigationarea in west Sichuan Province. Stations 1-7 are located in the Daxue Mountains and stations 8-10 are distributed in the Qionglai Mountains.
Jianguo Liu1,2*, Qian Liu1, Litao Zhang1,2, Chenlin Liu3, Wei Lin1, Xiaohang Huang3
1. Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China
2. Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao, China
3. The First Institute of Oceanography, State Oceanic Administration, Qingdao, China
* Corresponding author.
Xiaohang Huang, Tel: +86 532 82898709; E-mail: jgliu@qdio.ac.cn
Received 30-08-2017; received in revised form 07-02-2018; accepted 07-02-2018
Abstract The ecological distribution and physiological properties of a new variety of Trentepohlia aurea, an aerial microalga found in the west plateau klint of the Sichuan Basin, China, were investigated. This variety of T.aurea is widely distributed in the Daxue Mountainsand the Qionglai Mountains 1500-5000 m above sea level, which covers over 30 000 km2 in Wenchuan, XiaoJin, Danba, Daou, Kangding and Luding counties. The T. aurea variety grows naturally on stone surfaces, dotting the stone in an array of saffron yellow and brownish red color due to its high carotenoids (b-carotene mainly) accumulation. Carotenoids in this alga, accounting for 2.2% of its dry weight, can effectively protect the cells from photodamage in the high plateau area. The T.aurea variety also has a powerful water-absorptive capacity and can get enough water from drizzle or even from the moisture in fog. Although the photochemical reaction of the dessicated T.aurea was severely inhibited, its photochemical activity recovered fully in a second when the alga was re-hydrated. It also has a unique cold and freezing resistance. After being frozen at -20°C for 24 h, the alga still showed a high photochemical activity. Unlike the other isolates of Trentepohlia, this variety of T.aurea could not survive high temperature for a long time. Upon a temperature increase from 10°C to 30°C, both photosynthetic and respiratory rates in the T. aurea variety dropped sharply. The photosynthetic and respiratory rates at 25°C were only about 14% and 5.8% of that at 10°C, respectively. Based on above results, the T. aurea variety is tolerant of strong light, desiccation and cold. It has potential as a commercial source of natural b-carotene, and may be utilized as a bio-functional material for production of daily necessities because of its powerful water absorptive capacity
Keywords: b-carotene; photosynthesis; dehydration; rehydration; cold; Trentepohlia aurea
1. Introduction
After the strong earthquake in Wenchuan, May 12 2008, some mountain climbers paid much more attention to the west plateau klint of Sichuan Basin. An amateur climber visited our Yunnan Alphy Biotech Co. Ltd, a facility for culturing Haematococcus pluvialis for production of natural astaxanthin, in Yunnan, China and told us that there were lots of reddish stones in the mountains of west Sichuan. However, the reason why the rocks become reddish is still a riddle. People believe it is caused by some oxidized iron ore in the stone, or by some species of lichen growing on the stone surface. The local Tibetans have an old legend that the reddish color is the shed blood of four beautiful fairies that were killed by the demon Moerdo, a brutal and merciless failure as suitor.
The unicellular microalga H. pluvialis is a dominant species on the stones in intermittent shallow rain-water pools, usually color stones red because of its high accumulation of astaxanthin (Droop, 1954; Czygan,1970). Does H. pluvialis or some other microalgae distributed in the mountain range? We made an on-the-spot investigation followed by some eco-physiological studies in our Qingdao laboratory. The results are presented in this article.
2. Materials and methods
2.1 Material
2.2 DNA extraction and 18S rDNA cloning
After smashing thalli with liquid nitrogen, genomic DNA of the scraped red materials was extracted using DNA extract kit (Tiangen Biotech Co). PCR amplification of the 18S rDNA was performed using primers designed for green algae (Medlin et al. 1988), 18sF: AACCTGGTTGATCCTGCCAGT, 18sR: TGATCCTTCTGCAGGTTCACCTAC. PCR conditions were as described by Liu et al. (2006) with a 5 min hot start at 94°C, followed by 30 cycles of 2 min at 94°C, 1.5 min at 45°C and 2 min at 72°C. Amplified DNA was ligated into the pMD18-T vector and transformed into Escherichia coli strain DH5a. The positive clones containing 18S rDNA were pooled and sequenced by Casarray Co. Ltd., Shanghai, China.
Clustal X (Thompson et al., 1997) was used for sequence alignment. Most of the 18S rDNA sequences for Trentepohlia spp. found in GenBank were selected to construct a phylogenetic tree with the sequence of our strain. The tree was reconstructed on the 18S rDNA database using MAGE 3.1 (Kumar et al., 2004). Twenty-oneTrentepohlia taxa were used to build the data matrix with twoCladophoralean taxa as the outgroup with the neighbor-joining methods, following the recommendation of Lόpez-Bautista et al. (2006). Bootstrap with 1000 replications (Felsenstein, 1985) was used to assess the support of nodes.
Pigments of the scraped red material were extracted in80% acetone and determined by traditional spectrophotometry as described by Arnon (1949).
2.5 Photosynthetic and respiratory rate measurements
The photosynthetic O2 evolution rate (photosynthetic rate) and consumption rate (respiratory rate) in response to a temperature gradient (30, 25, 19, 16, and 10°C) were measured in an OxyLab-2 liquid-phase oxygen electrode system (Hansatech Instruments, Norfolk, UK) as per Padmasree and Raghavendra (1999). For the raw scraped red material rewetted by water, respiratory rate was measured in the dark. Photosynthetic rate was measured at a PFD of 500 μmol photons·m-2·s-1. Two milliliter of reaction medium for photosynthetic assay and respiratory rate contained 1 mM NaHCO3.
2.6 Chlorophyll a fluorescence (OJIP) transient measurement
Chlorophyll a fluorescence (OJIP) transients of the T. aurea variety were measured in each stage of dehydration and rehydration at different temperatures (from -20°C to 25°C)using a Handy-PEA (Hansatech Instruments, Norfolk, UK) as per Zhang et al. (2011) after 20-min dark adaptation. To obtain dry samples, raw scraped red material was room dried at 30% relative air humidity for 48 h. The OJIP transients were induced by red light (650 nm wavelength, 2000 μmol·m-2·s-1) provided by an array of three light-emitting diodes (LEDs, peak 650 nm).
3.1 Distribution of mossy“red”or “orange”rocks
During our trip in the mid-April, 2009 to the Daxue Mountains and Qionglai Mountains in west Sichuan, mossy “red” or “orange” rocks were found in many stations (Fig.1)widely seen in river banks of the Yanzi Gou (Swallow Vale)and the Hailuo Gou (Snail Vale) in Luding County, the Taizhan Valein Daofu County, the Maoniu Gou (Yak Vale)in Danba County, the Shuangqiao Gou (Double Bridge Vale)in Xiaojin County, and some snow covered mountain areas. In fact, these“red” or “orange” rocks are mossed with Trentepohlia spp. and distributed widely for several kilometersacross thesevalleys and snow covered areas (Fig.2).

Fig.1 The investigationarea in west Sichuan Province. Stations 1-7 are located in the Daxue Mountains and stations 8-10 are distributed in the Qionglai Mountains.
Such colorful rocks spot the local lands like blooming flowers, making the areasas the “gallery of landscapes” (Fig.2), inducing thenatural reserves forpanda and golden monkeyin Wolong of Wenchuan Countyand in the western part of Kangding County. The altitude of these places varied from 1500 to 5000 m above sea level. The local climate changed frequently from heavy snow, drizzle, and dense fog, to cloudy and sunny days. The temperature we measured during daytime in April varied from 16°C to - 4.0°C. The linear distances between the west and the east sites where the colorful rocks were found during this trip is about 180 km, while that between the south and north sites is about 170 km. Our filed investigation showed that the area of natural distribution of these rocks was more than 30 000 km2. We believe that in neighboring areas in Sichuan and Tibet there would be a much greater distribution area of such rocks. Interestingly, these rocks were usually found in the places out of water but water pockets and pools.

Fig.2 The natural distribution of red stones in the snow-covered mountains and valleys.
3.2 Microscopic observation and molecular analysis
The scraped samples became brittle after being dried. The friable dried material could be crumbled easily by hand. However, it showed a strong water absorbance ability when water drops added. Microscopic observation (Fig.3) showed that all the scraped red samples were a filamentous alga consisting of chains of 10 to 20 cells that regularly or irregularly shaped and embedded in clear mucilage. Although a few green algal cells were present, most cells in the filamentous alga were filled with orange-pigmented oil droplets of various sizes. In some samples, cells seemed to have lost their green chloroplasts completely and contained only orange colored oil droplets (Fig.3). From these observations, the alga collected from the colorful rocks was identified as aerial microalga of the genus Trentepohlia (Chlorophyceae).
![]()
![]()

Fig.3 The aerial filaments of carotenoid-enriched Trentepohlia aurea variety. The raw scraped samples were rewetted by a drop of water for microscopic examination.
The two phylogenetic linages of Trentepohlia were correlated with that in Lόpez-Bautista’s phylogenetic tree including six genera (Cephaleuros, Phycopeltis, Physolinum, Printzina, Stomatochroon, and Trentepohlia) of the Trentepohliales. T.arborum,T. aurea,and T. dialeptaform the first group, and T. iolithus, T. abietina, and T. umbrina from another well-defined monophyletic group. In the phylogentic tree, the algal strain isolated from west Sichuan (termed strain 1 in Fig.4) was clustered with T. aurea in a bootstrap value of 72%. Thus, according to the phylogenetic analysis of 18S rDNA, strain 1 is aT. aurea variety.

Fig.4 Neighbor-joining tree resulting from analysis of 18S rDNA sequences for 21 Trentepohlia taxa. The 18S rDNA sequence had been submitted to GenBank under accession number MG831570. Support values more than 50% are shown on the branches.
The array of saffron yellow and brownish red colors of the stones was due to the high concentration of carotenoids (b-carotene mainly) found in the algae growing on rocks. Our data show the chlorophyll and carotenoid content in the T. aurea variety were 6.67 and 22.01g kg-1dry-weight, respectively. The high carotenoid׃chlorophyll ratio of the T. aurea variety (3.3׃1) reflected its adaptation protecting against the environmental stresses (strong insolation and UV level) in the high mountains.
3.4 Photosynthetic and respiratory activities of the alga when exposed to different stresses
The effects of temperature on photosynthetic and respiratory activities of the T. aurea variety were evaluated by measurement of O2 evolution and consumption rates (Fig.5a, b). When temperature varied between 19°C and 30°C, the respiratory rate of the T. aurea variety remained at a relatively low level (about 180 nmol O2 min-1·g-1FW) (Fig.5a). However, the respiratory rate of the T. aurea variety sharply increased once the temperature dropped to 16°C and below (Fig.5a). The net O2 evolution rate of the T. aurea variety linearly increased as the temperature decreased from 30°C to 10°C (Fig.5b). The above results reveal that the basic metabolism of this T. aurea variety become much more active when exposed to a low temperature. Unlike other Trentepohlia species, this T. aureais a cold-resistant variety. Therefore, a high photosynthetic activity of the new variety was expected when it was exposed to a very cold, even frozen, environmental condition.
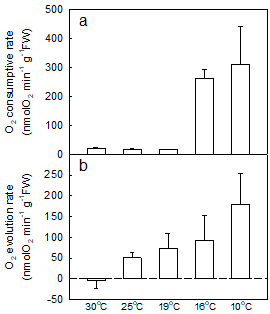
Fig.5 Respiratory rates (a) and net photosynthetic O2 evolution rates (b) of the T. aurea variety in response to temperature
3.5 Chlorophyll a fluorescence (OJIP) transients of the T. aurea variety exposed to different stresses
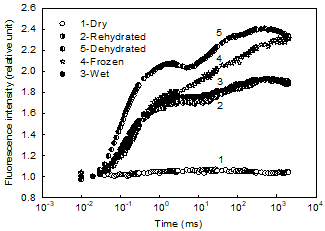
Fig.6 The chlorophyll a fluorescence (OJIP) transients of the T. aurea variety under different conditions
The photosynthetic characteristics of the T. aurea variety exposed to different conditions were studied by analyzing the chlorophyll a fluorescence (OJIP) transients. The difference in OJIP transients indicate that the primary photosynthetic photochemical activity of the T. aurea varied significantly in each stage of dehydration and rehydration. The photochemical activity in fully dried T. aurea was completely lost (Fig.6, curve 1). The dormant photosynthesis recovered quickly when the dried T. aurea was wetted even for dry algal material soaked in water for just 1 s (Fig.6, curve 2). No significant difference of the OJIP transients was found in the rehydrated T. aurea (Fig.6, curve 2) and fully water-soaked material (Fig.6, curve 3). During the process of dehydration, the OJIP transient (Fig.6, curve 5) had a significant increase compared to that of water-soaked material, showing that this aerial alga had its highest photochemical activity at the stage of half dryness. The OJIP transient of this alga under a freezing environmental condition (-20°C) was significantly increased (Fig.6, curve 4) compared to that of wet material at a moderate room temperature. Therefore, a very high photochemical activity in this variety of T. aurea, when frozen, was expected.
The morphological criteria traditionally used for the circumscription of genera and species of Trentepohliales in many cases did not match the phylogenetic patterns (Lόpez-Bautista et al., 2006). It is difficult to distinguish T. aurea from other species such as T. abietina in morphology. However, T. aurea and T. abietina occur in different lineages in phylogeny, indicating a remarkable separation. Therefore, a reconsideration of the morphological features used for the identification of these species (and presumably other species of Trentepohlia) is necessary. Based on our results of 18S rDNA sequences (Fig.4), two phylogentic linages of Trentepohlia we observed were correlated with those in the phylogenetic tree of Lόpez-Bautista et al. (2006).Ouralgal strain is clustered with three 18S ribosomal RNA genes including DQ399590, AB110783, and AY052567. Sequence alignment among them shows a high sequence similarity among these four sequences (higher than 99%) including the highest onesMG831570 and DQ399590 (100% sequence similarity, data not shown).
Earlier studies indicate that Trentepohlia species occurred mainly in tropical and sub-tropical regions and grew on concrete and rock surfaces and even on trees. Thus, Trentepohlia is usually adapted to high and moderate temperatures (Abe, 1998; Akiyama, 1961;Sarma, 1986). Abe et al. (1998) reported that the maximum photochemical activity of T. aurea was in the range of 30 °C to 35 °C. However, for the Sichuan T. aurea, both the unexpected decrease in photosynthetic rate with increases of incubation temperature (Fig.5b), and the high photochemical activity when the alga was exposed to -20 °C (Fig.6, curve 4) indicate that this T. aurea has a high tolerance and adaptability to cold and freezing environments. In contract to the common concept of respiration increasing with rising temperature, the respiratory rate in this T. aurea uniquely increased with decreasing temperature (Fig.5a). This may be a special adaptive mechanism for this variety of T. aurea, living and surviving by generating additional energy for maintaining its cell metabolism in a cold and even freezing condition of their mountain habitats at a high altitude. Based on the above results, we consider this aerial T. aurea in Sichuan a new variety of T. aurea that totally differs from other Trentepohlia isolates.
Although the photochemical activity in T. aurea was completely lost when the aerial alga was fully dried (Fig.6, curve 1), the photochemical activity recovered quickly almost immediately after the dried T. aurea got wet. These results illustrate that both the rehydration ability and the activity revival in this variety of T. aurea were surprisingly strong. These T. aurea habitats are subject to drying and rewetting cycles caused by changes in weather. The T. aurea variety has a powerful water absorptive capacity and therefore can get enough water from drizzle or even from moist fog. The T. aurea variety is tolerant to desiccation because the filaments are able to recover physiological performance, especially photosynthesis, quickly when rewetted after short periods of desiccation.
During the process of dehydration, the photochemical activity increased significantly (Fig.6, curve 5) compared to that of water-soaked material, which shows that this aerial alga had its highest photosynthetic activity at the stage of half dryness. In other words, this variety of T. aurea under fully water-soaked conditions would not grow as well as in dry but humid conditions. This observation may explain the distribution of “red” rocks found on the ground, and none in aqueous environments such as water pools, riverbeds, and streams with flowing water.
It is known that both β-carotene and astaxanthin have antioxidant activity, and thus provide a cellular defense against reactive oxygen species (Ben-Amotz et al., 1983; Liu et al., 2007; Liu et al., 2010). The halotolerant green alga Dunaliella can accumulate large amounts of β-carotene under appropriate conditions, such as high light intensity, high temperature, high salinity, and nitrate deficiency (Borowitzka et al. 1984; Liu et al. 1996). Dunaliella bardawil contains at least 8% β-carotene on dry weight basis under stress conditions (e.g., high light intensity and high salt concentration) (Ben-Amotz et al., 1983). In the present study, photochemical activity in T. aurea was completely lost when the alga was fully dried (Fig.6, curve 1). In this state, the aerial alga was highly subject to photoinhibition because chlorophyll continued to absorb photosynthetically active radiation, even though the chemical reactions of photosynthesis were stalled and the excitation energy could not be used by photosynthesis. Such algae need effective protective mechanisms to allow harmless dissipation of the excess excitation energy (Heber et al., 2000; 2001; 2006a, b; Gray et al., 2007). It appears that the accumulation of carotenoids (β-carotene mainly) in the alga is its way to dissipate the excess energy under such conditions. Carotenoids in the T. aurea make up 2.2% of dry its weight, which could likely effectively protect the cells from photodamage in the high-light and high plateau area.
In conclusion, T. aurea is tolerant to strong light, desiccation, and cold environment. It has potential as a commercial source of natural b-carotene. It may also be utilized as a bio-functional material for production of daily necessities, such as infant diapers and sanitary napkins because of its great water absorptive capacity.
This work is financially supported by the National Nature Science Foundation of China (Nos. 31300330 and31572639). The authors would like to thank Mr. ZHANG Yong from Yunnan Alphy Biotech Co.Ltd for his arrangement of the unforgettable trip in the central Sichuan. Special thanks are given to Dr. John van der Meer, a former President of the Phycological Society of America and Director of Research at the NRC Institute for Maine Biosciences in Halifax, Canada, for his assistance with proofreading.
References
Abe K, Mihara H, Hirano M. (1998). Characteristics of growth and carotenoid accumulation of the aerial microalga Trentepohliaaurea in liquid culture. J. Mar. Biotechnol. 6:53-58.
Akiyama M. (1961). Aerial and terrestrial algae in San-in Region of Honshû, Japan Bull Shimane Univ (Nat Sci) 10:75-89.
Arnon DI. (1949). Copper enzymes in isolated chloroplasts, polyphenol oxidase in Beta vulgaris, Plant Physiology 24:1-5.
Ben-Amotz A, Avron M. (1983). On the factors which determine massive carotene accumulation in the halotolerant alga Dunaliellabardawil. Plant Physiol 72:593-597.
Borowitzka LJ, Borowitzka MA, Moulton TP. (1984). The mass culture of Dunaliellasalina for fine chemicals: from laboratory to pilot plant. Hydrobiologia 116/117:115-121.
Czygan FC. (1970). Blood-rain and blood-snow: Nitrogen-deficient cells of Haematococcuspluvialis and Chlamydomonasnivalis. Arch Microbiol. 74:69-76.
Droop MR. (1954). Conditions governing haematochrome formation and loss in the alga Haematococcuspluvialis Flotow. Arch Microbiol. 20:391-397.
Felsenstein J. (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783-791.
Gray DW, Lewis LA, Cardon ZG. (2007). Photosynthetic recovery following desiccation of desert green algae (Chlorophyta) and their aquatic relatives. Plant, Cell & Environment 30: 1240-1255.
Heber U, Bilger W, Bligny R, et al. (2000). Phototolerance of lichens, mosses and higher plants in an alpine environment: analysis of photoreactions. Planta 211:770-780.
Heber U, Bilger W, Shuvalov VA. (2006a). Thermal energy dissipation in reaction centres and in the antenna of photosystem II protects poikilohydric mosses against photo-oxidation. Journal of Experimental Botany 57:2993-3006.
Heber U, Bukhov NG, Shuvalov VA, et al. (2001). Protection of the photosynthetic apparatus against damage by excessive illumination in homoiohydric leaves and poikilohydric mosses and lichens. Journal of Experimental Botany 52:1999-2006.
Heber U, Lange OL, Shuvalov VA. (2006b). Conservation and dissipation of light energy as complementary processes: homoiohydric and poikilohydric autotrophs. Journal of Experimental Botany 57:1211-1223.
Kumar S, Tamura K, Nei M. (2004). MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Briefings in Bioinformatics 5:150-163.
Liu CL, Huang XH, Wang XL, et al. (2006). Phylogenetic studies on two strains of Antarctic ice algae based on morphological and molecular characteristics. Phycologia 45:190-198.
Liu JG, Pan LS, Wei XL, et al. (2007). Physiological behaviors of Wistar rat supplemented with astaxanthin-enriched Haematococcuspluvialis powder. Food and Drug 12:26-30.
Liu JG, Wu CY, Chen NH, et al. (1996). Effect of nitrate and phosphate on accumulation of β-carotene isomers in Dunaliellasalina. Chin J Ocean Limn, 14:165-169.
Liu JG, Zhang XL, Sun YH, et al. (2010). Antioxidative capacity and enzyme activity in Haematococcuspluvialis cells exposed to superoxide free radicals. Chin J Ocean Limn 28:1-9.
Lόpez-Bautista JM, Rindi F, Guiry MD. (2006). Molecular systematics of the subaerial green algal order Trentepohliales: an assessment based on morphological and molecular data. International Journal of Systematic and Evolutionary Microbiology 56:1709-1715.
Medlin L, Elwood HJ, Stickel S, et al. (1988). The characterization of enzymatically amplified eukaryotic 16S-like rRNA coding regions. Gene 71:491-499.
Merzlyak MN, Solovchenko AE, Gitelson AA. (2003). Reflectance spectral features and non-destructive estimation of chlorophyll, carotenoid and anthocyanin content in apple fruit. Post Biol Technol 27:197-211.
Padmasree K, Raghavendra AS. (1999). Response of photosynthetic carbon assimilation in mesophyll protoplasts to restriction on mitochondrial oxidative metabolism: Metabolites related to the redox status and sucrose biosynthesis. Photosynthesis Research 62:231-239.
Sarma P. (1986). The freshwater Chaetophorales of New Zealand. Beihefte zur Nova Hedwigia 58:1-169.
Sims DA, Gamon JA. (2002). Relationship between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and development stages. Remote Sens Environ 81:337-354.
Thompson JD, Gibson TJ, Plewniak F. (1997). The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25:4876-4882.
Zhang LT, Zhang ZS, Gao HY, et al. (2011). Mitochondrial alternative oxidase pathway protects plants against photoinhibition by alleviating inhibition of the repair of photodamaged PSII through preventing formation of reactive oxygen species in Rumex K-1 leaves. Physiologia Plantarum 143:396-407.